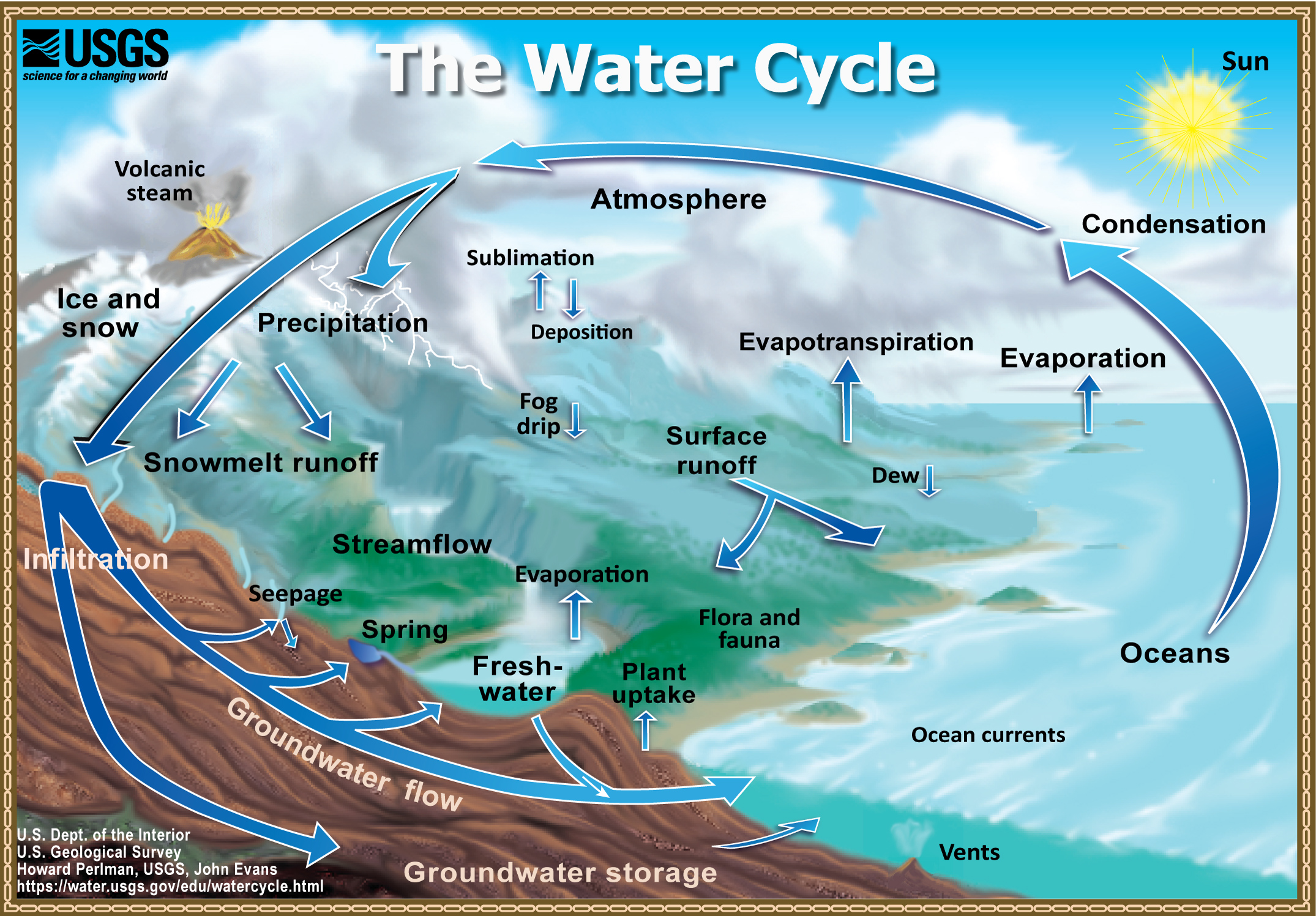
What Is The Parts Of Water . It is composed of the following:. Of the many processes involved in the water cycle, the most important are. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Because of the higher electronegativity. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them;
from water.usgs.gov
Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Because of the higher electronegativity. It is composed of the following:. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Of the many processes involved in the water cycle, the most important are. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found.
Evaporation, The Water Cycle, from USGS WaterScience School
What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. It is composed of the following:. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Of the many processes involved in the water cycle, the most important are. Because of the higher electronegativity. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Is The Parts Of Water Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. It is composed of the following:. Because of the higher electronegativity. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Of the many processes. What Is The Parts Of Water.
From www.slideserve.com
PPT Groundwater and the Hydrologic Cycle PowerPoint Presentation What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. It is composed of the following:. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Most water contains varying. What Is The Parts Of Water.
From inspectapedia.com
Water Softener Iron Stain & Sediment Cleaning What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. It is composed of the following:. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons. What Is The Parts Of Water.
From www.metlink.org
MetLink Royal Meteorological Society The Changing Water Cycle What Is The Parts Of Water It is composed of the following:. Of the many processes involved in the water cycle, the most important are. Because of the higher electronegativity. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared. What Is The Parts Of Water.
From bottlefirst.com
Water Bottle Parts Name Find Out Here! What Is The Parts Of Water Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended. What Is The Parts Of Water.
From ambitiousmares.blogspot.com
31 Label The Water Cycle Labels Design Ideas 2020 What Is The Parts Of Water Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. The different. What Is The Parts Of Water.
From waterfilterguru.com
UV Water Filter Diagram (Quick View of All Components) What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Of the many processes involved in the water cycle, the most important are. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Because of the higher electronegativity.. What Is The Parts Of Water.
From fun2learnworksheets.com
Label the Water Cycle Free Worksheet STEM CLIL ESL Fun2Learn What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Water is a simple molecule consisting of one oxygen atom. What Is The Parts Of Water.
From www.ganjing.com
Why parts of America are 'certainly in a water crisis' and what can be What Is The Parts Of Water Because of the higher electronegativity. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. It is composed of the following:. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Most water contains varying amounts of. What Is The Parts Of Water.
From design.udlvirtual.edu.pe
How Does A Basic Water Pump Work Design Talk What Is The Parts Of Water Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; It is composed of the following:. Of the many. What Is The Parts Of Water.
From boilersinfo.com
Water Tube Boiler Parts and Functions What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. It is composed of the following:. The different components of the. What Is The Parts Of Water.
From www.angi.com
The Parts of a Toilet You Need to Know What Is The Parts Of Water Of the many processes involved in the water cycle, the most important are. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Water is a simple molecule consisting. What Is The Parts Of Water.
From www.researchgate.net
The components of water supply system (WSS). Picture adapted from QEPA What Is The Parts Of Water Of the many processes involved in the water cycle, the most important are. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The different. What Is The Parts Of Water.
From reviewmotors.co
Pool Filter Parts Names Reviewmotors.co What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. In water, each hydrogen nucleus is bound to the central oxygen. What Is The Parts Of Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? What Is The Parts Of Water Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Of the many. What Is The Parts Of Water.
From drlogy.com
Total Body Water Calculator Ideal Body Water Intake Drlogy What Is The Parts Of Water In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; It is composed of the following:. Because of the higher electronegativity. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. The different components of the hydrosphere vary. What Is The Parts Of Water.
From www.storyboardthat.com
Label the Parts of the Water Cycle Storyboard por templates What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Of the many processes involved in the water cycle, the most important are. It is composed of the following:. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between. What Is The Parts Of Water.
From www.myxxgirl.com
Draw A Labelled Diagram Showing The Water Cycle Environmental My XXX What Is The Parts Of Water In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Of the many processes involved in the water cycle, the most important are. Properties of water include its. What Is The Parts Of Water.
From einvoice.fpt.com.vn
Parts Of A Toilet And How It Works (3 Detailed Diagrams), 42 OFF What Is The Parts Of Water Because of the higher electronegativity. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of. What Is The Parts Of Water.
From www.freepik.com
Premium Vector Water cycle diagram Earth hydrologic process What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Properties. What Is The Parts Of Water.
From www.linquip.com
Parts of Water Pump Industrial Manufacturing Blog linquip What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Because of the higher electronegativity. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; It is composed of the following:. The different components of the hydrosphere vary. What Is The Parts Of Water.
From bottlefirst.com
Water Bottle Parts Name What Is The Parts Of Water Of the many processes involved in the water cycle, the most important are. It is composed of the following:. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity.. What Is The Parts Of Water.
From ar.inspiredpencil.com
Name Parts On A Vessel What Is The Parts Of Water Of the many processes involved in the water cycle, the most important are. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as. What Is The Parts Of Water.
From toolkit.climate.gov
Water Cycle U.S. Climate Resilience Toolkit What Is The Parts Of Water Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Of the many processes involved in the water cycle, the most important are. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Properties of water include its chemical formula h2o, density, melting, boiling. What Is The Parts Of Water.
From sinaumedia.com
Laboratory Water Bath Functions and Working Principles Sinaumedia What Is The Parts Of Water In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Of the many processes involved in the water cycle, the most important are. It is composed of. What Is The Parts Of Water.
From pmm.nasa.gov
The Water Cycle Precipitation Education What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has. What Is The Parts Of Water.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples What Is The Parts Of Water Of the many processes involved in the water cycle, the most important are. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; The different components of the hydrosphere vary in their. What Is The Parts Of Water.
From microbeonline.com
Water Bath Parts, Principle, and Applications • Microbe Online What Is The Parts Of Water In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Of the many processes involved in the water cycle, the most important are. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to. What Is The Parts Of Water.
From www.researchgate.net
(a) Components of singlejet water meter [4 e] Download Scientific What Is The Parts Of Water In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Of the. What Is The Parts Of Water.
From water.usgs.gov
Evaporation, The Water Cycle, from USGS WaterScience School What Is The Parts Of Water In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Of the many processes involved in the water cycle, the most important are. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to. What Is The Parts Of Water.
From www.vrogue.co
Three Diagrams Showing The Different Stages Of Water vrogue.co What Is The Parts Of Water Because of the higher electronegativity. The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Most water contains varying amounts. What Is The Parts Of Water.
From www.smokecartel.com
Infographic Anatomy Of A Water Pipe Smoke Cartel What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Of the many processes involved in the water cycle, the most important are. In water, each hydrogen nucleus is bound to the central. What Is The Parts Of Water.
From removeandreplace.com
Bottled Water Cooler Parts Accessories For Bottled Water Dispensers What Is The Parts Of Water The different components of the hydrosphere vary in their state (solid, liquid, or gas) and the location where they are found. Of the many processes involved in the water cycle, the most important are. Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a. What Is The Parts Of Water.
From heilplumbingdmv.com
The 7 Critical Parts of a Water Heater (Electric) What Is The Parts Of Water It is composed of the following:. Because of the higher electronegativity. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; Properties of water include its chemical formula. What Is The Parts Of Water.
From mcwaneductile.com
Two Key Components of Water Systems Directly Related to Pipeline What Is The Parts Of Water Properties of water include its chemical formula h2o, density, melting, boiling point & how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Most water contains varying amounts of dissolved minerals and salts, plus an abundance of suspended particles such as silt and. The different components of the hydrosphere vary in their state (solid,. What Is The Parts Of Water.