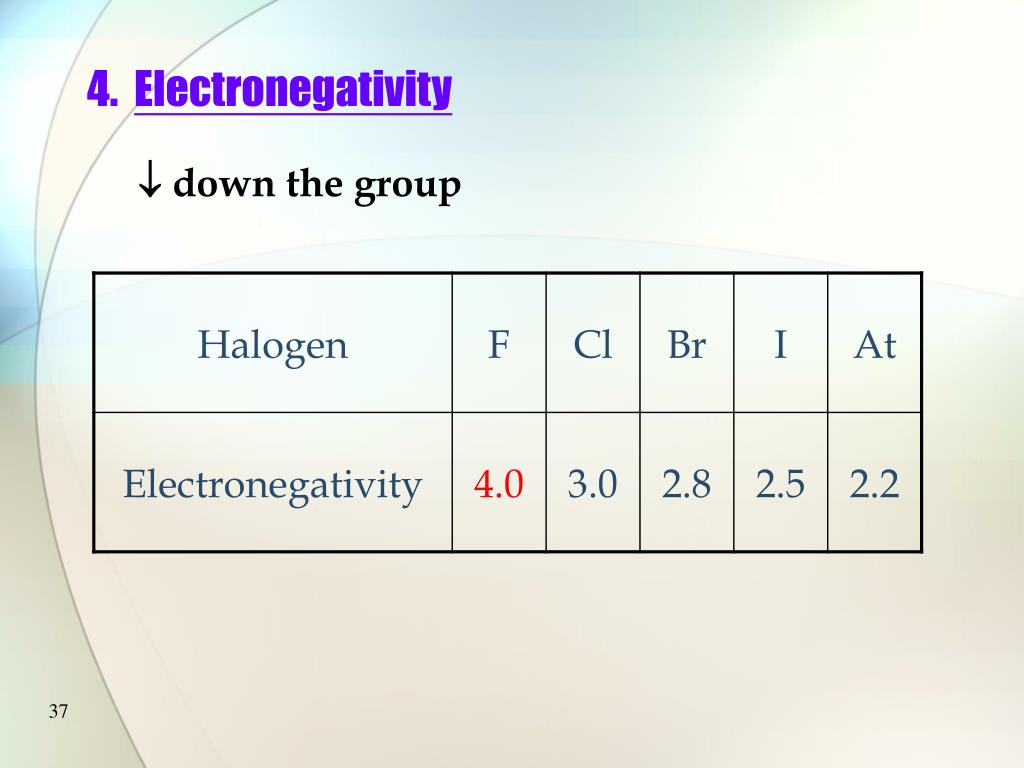
Properties Of Halogens In Water . This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Physical properties of the halogens. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Fluorine, chlorine, bromine and iodine. Placed in a vertical column on the right of the. These reactive nonmetals have seven valence electrons. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens are located on the left of the noble gases on the periodic table. The group 7 elements are called halogens. As a group, halogens exhibit highly variable physical properties.
from www.slideserve.com
Fluorine, chlorine, bromine and iodine. The halogens are located on the left of the noble gases on the periodic table. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Physical properties of the halogens. As a group, halogens exhibit highly variable physical properties. These reactive nonmetals have seven valence electrons. Placed in a vertical column on the right of the. The group 7 elements are called halogens.
PPT Characteristic Properties of the Halogens PowerPoint Presentation
Properties Of Halogens In Water Placed in a vertical column on the right of the. Physical properties of the halogens. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). As a group, halogens exhibit highly variable physical properties. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. The halogens are located on the left of the noble gases on the periodic table. These reactive nonmetals have seven valence electrons. Fluorine, chlorine, bromine and iodine. Placed in a vertical column on the right of the. The group 7 elements are called halogens. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens):
From exojwcmhn.blob.core.windows.net
Properties Of Alkali Metals Halogens And Noble Gases at Jean Myrick blog Properties Of Halogens In Water The group 7 elements are called halogens. Fluorine, chlorine, bromine and iodine. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. This page. Properties Of Halogens In Water.
From www.thoughtco.com
Halogen Elements and Properties Properties Of Halogens In Water Placed in a vertical column on the right of the. As a group, halogens exhibit highly variable physical properties. Physical properties of the halogens. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Fluorine, chlorine, bromine and iodine. The halogens are located on the left of the noble gases on the periodic table. The halogens have. Properties Of Halogens In Water.
From scienceinfo.com
Chemical Properties of Halogen Elements and Hydrogen Halides Properties Of Halogens In Water These reactive nonmetals have seven valence electrons. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. The halogens are located on the left of the noble gases on the periodic table. As a group, halogens exhibit highly variable physical properties. Fluorine, chlorine, bromine and iodine. Some chemical and physical properties of the halogens are summarized. Properties Of Halogens In Water.
From es.slideshare.net
Halogens part 1 physical properties Properties Of Halogens In Water Fluorine, chlorine, bromine and iodine. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Placed in a vertical column on the right of the. As a group, halogens exhibit highly variable physical properties. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). These reactive nonmetals have seven valence electrons. Physical properties. Properties Of Halogens In Water.
From www.thoughtco.com
Halogen Elements and Properties Properties Of Halogens In Water As a group, halogens exhibit highly variable physical properties. Fluorine, chlorine, bromine and iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens are located on the left of the noble gases on the periodic table.. Properties Of Halogens In Water.
From www.science-revision.co.uk
The halogens Properties Of Halogens In Water The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Fluorine, chlorine, bromine and iodine. These reactive nonmetals have seven valence electrons. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens. Properties Of Halogens In Water.
From exojwcmhn.blob.core.windows.net
Properties Of Alkali Metals Halogens And Noble Gases at Jean Myrick blog Properties Of Halogens In Water Fluorine, chlorine, bromine and iodine. The halogens are located on the left of the noble gases on the periodic table. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): As a group, halogens exhibit highly variable physical properties. The group 7 elements are called halogens. Placed in a vertical column on. Properties Of Halogens In Water.
From issr.edu.kh
Halogens Properties Of Halogens In Water As a group, halogens exhibit highly variable physical properties. The group 7 elements are called halogens. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Placed in a vertical column on the right of the. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens act a good oxidising agents. Properties Of Halogens In Water.
From byjus.com
Elements Of Halogen Group Properties Halogen Elements And List Properties Of Halogens In Water Fluorine, chlorine, bromine and iodine. As a group, halogens exhibit highly variable physical properties. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. The group 7 elements are called halogens. Physical properties of the halogens. Placed in a vertical column on the right of the. The halogens are located on. Properties Of Halogens In Water.
From www.researchgate.net
Some properties of the Halogens Download Table Properties Of Halogens In Water Placed in a vertical column on the right of the. The group 7 elements are called halogens. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens are located on the left of the noble gases on the periodic table. These reactive nonmetals have seven valence electrons. The halogens act a good oxidising agents as. Properties Of Halogens In Water.
From newtondesk.com
Halogens (Periodic Table) Properties, Uses, & Facts Properties Of Halogens In Water These reactive nonmetals have seven valence electrons. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Placed in a vertical column on the right of the. The group 7 elements are called halogens. Some chemical and physical. Properties Of Halogens In Water.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2319114 Properties Of Halogens In Water Physical properties of the halogens. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Placed in a vertical column on the right of the. The halogens are located on the left of the noble gases on the periodic table. The group 7 elements are called halogens. These reactive nonmetals have seven. Properties Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogens In Water The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Placed in a vertical column on the right of the. As a group, halogens exhibit highly variable physical properties. These reactive nonmetals have seven valence electrons. The group 7 elements are called halogens. This page discusses the trends in the atomic. Properties Of Halogens In Water.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation, free download Properties Of Halogens In Water The halogens are located on the left of the noble gases on the periodic table. Physical properties of the halogens. Fluorine, chlorine, bromine and iodine. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. The group 7 elements are called halogens. The halogens have uses in water purification and as. Properties Of Halogens In Water.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2319114 Properties Of Halogens In Water Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Placed in a vertical column on the right of the. Fluorine, chlorine, bromine and iodine. Physical properties of the halogens. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): As a group, halogens exhibit highly variable physical properties.. Properties Of Halogens In Water.
From www.slideserve.com
PPT Physical properties of halogens PowerPoint Presentation, free Properties Of Halogens In Water The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Placed in a vertical column on the right of the. Physical properties of the halogens. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The group 7 elements are called halogens. Fluorine, chlorine, bromine and iodine. The. Properties Of Halogens In Water.
From chem.libretexts.org
Group 17 Physical Properties of the Halogens Chemistry LibreTexts Properties Of Halogens In Water These reactive nonmetals have seven valence electrons. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Fluorine, chlorine, bromine and iodine. This page discusses the trends in the atomic and physical properties of the group 7. Properties Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogens In Water Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Placed in a vertical column on the right of the. Physical properties of the halogens. Fluorine, chlorine, bromine and iodine. As a group, halogens exhibit highly variable physical properties. These reactive nonmetals have seven valence electrons. The halogens have uses in water purification and as bleaching agents. Properties Of Halogens In Water.
From www.pinterest.com
Pin by M B on Chemistry Chemical equation, Hydrogen chloride, Chemistry Properties Of Halogens In Water Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Physical properties of the halogens. Fluorine, chlorine, bromine and iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Placed in a vertical column on the right of the. The halogens are located on the left of the. Properties Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogens In Water Placed in a vertical column on the right of the. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): The halogens have uses in water purification and as bleaching agents (chlorine), as flame. These reactive nonmetals have seven valence electrons. As a group, halogens exhibit highly variable physical properties. The halogens. Properties Of Halogens In Water.
From www.chemicals.co.uk
Chemistry Group 7(17), The Halogens Properties Of Halogens In Water These reactive nonmetals have seven valence electrons. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. As a group, halogens exhibit highly variable physical properties. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Physical properties of the halogens. This page discusses the trends in the atomic and physical properties of. Properties Of Halogens In Water.
From www.youtube.com
The properties and reactions of the halogens YouTube Properties Of Halogens In Water Placed in a vertical column on the right of the. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. The halogens are located on the left of the noble gases on the periodic table. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. As a. Properties Of Halogens In Water.
From www.linstitute.net
AQA A Level Chemistry复习笔记2.3.2 Chemical Properties of Group 7翰林国际教育 Properties Of Halogens In Water Physical properties of the halogens. As a group, halogens exhibit highly variable physical properties. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Fluorine, chlorine, bromine and iodine. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. The group 7 elements are called halogens. Some. Properties Of Halogens In Water.
From www.slideserve.com
PPT Chapter 22 Chemistry of the Nonmetals PowerPoint Presentation Properties Of Halogens In Water This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): These reactive nonmetals have seven valence electrons. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. As a group, halogens exhibit highly variable physical properties. The halogens act a good oxidising agents as they accept electrons from. Properties Of Halogens In Water.
From ppt-online.org
Elements 17 (7A) group. Study of the properties of halogens and the Properties Of Halogens In Water Physical properties of the halogens. Placed in a vertical column on the right of the. The group 7 elements are called halogens. As a group, halogens exhibit highly variable physical properties. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Some chemical and physical properties of the halogens are summarized in. Properties Of Halogens In Water.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5949337 Properties Of Halogens In Water The group 7 elements are called halogens. The halogens are located on the left of the noble gases on the periodic table. Placed in a vertical column on the right of the. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Physical properties of the halogens. Fluorine, chlorine, bromine and iodine.. Properties Of Halogens In Water.
From www.pinterest.com
"All in the Family" Properties of Halogens Teaching chemistry Properties Of Halogens In Water The halogens have uses in water purification and as bleaching agents (chlorine), as flame. The halogens are located on the left of the noble gases on the periodic table. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): These reactive nonmetals have seven valence electrons. Placed in a vertical column on. Properties Of Halogens In Water.
From www.hanlin.com
AQA A Level Chemistry复习笔记2.3.1 Physical Properties of Group 7翰林国际教育 Properties Of Halogens In Water Physical properties of the halogens. The halogens are located on the left of the noble gases on the periodic table. These reactive nonmetals have seven valence electrons. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. This page discusses the trends in the atomic and physical properties of the group. Properties Of Halogens In Water.
From www.slideserve.com
PPT The following physical properties of the halogens appearance and Properties Of Halogens In Water As a group, halogens exhibit highly variable physical properties. Placed in a vertical column on the right of the. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. These reactive nonmetals have seven valence electrons. The halogens are located on the left of the noble gases on the periodic table. Some chemical and physical properties. Properties Of Halogens In Water.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Properties Of Halogens In Water The group 7 elements are called halogens. Physical properties of the halogens. These reactive nonmetals have seven valence electrons. Placed in a vertical column on the right of the. The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. The halogens have uses in water purification and as bleaching agents (chlorine),. Properties Of Halogens In Water.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Properties Of Halogens In Water The halogens act a good oxidising agents as they accept electrons from the species being oxidised and are reduced. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens have uses in water purification and as bleaching agents (chlorine), as flame. Physical properties of the halogens. As a group, halogens exhibit highly variable physical properties.. Properties Of Halogens In Water.
From www.sliderbase.com
Group 7 Halogens Presentation Chemistry Properties Of Halogens In Water The halogens are located on the left of the noble gases on the periodic table. The group 7 elements are called halogens. Fluorine, chlorine, bromine and iodine. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). Physical properties of the halogens. Placed in a vertical column on the right of the. This page discusses the trends. Properties Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Properties Of Halogens In Water These reactive nonmetals have seven valence electrons. The group 7 elements are called halogens. Physical properties of the halogens. Placed in a vertical column on the right of the. The halogens have uses in water purification and as bleaching agents (chlorine), as flame. As a group, halogens exhibit highly variable physical properties. The halogens act a good oxidising agents as. Properties Of Halogens In Water.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.2.1 Group 7 (Halogens)翰林国际教育 Properties Of Halogens In Water Fluorine, chlorine, bromine and iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): These reactive nonmetals have seven valence electrons. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). The halogens act a good oxidising agents as they accept electrons from the species being oxidised and. Properties Of Halogens In Water.
From www.pinterest.com
Pin on Periodic Table Halogen Properties Of Halogens In Water Physical properties of the halogens. Some chemical and physical properties of the halogens are summarized in table \(\pageindex{1}\). As a group, halogens exhibit highly variable physical properties. Placed in a vertical column on the right of the. These reactive nonmetals have seven valence electrons. The halogens are located on the left of the noble gases on the periodic table. Fluorine,. Properties Of Halogens In Water.