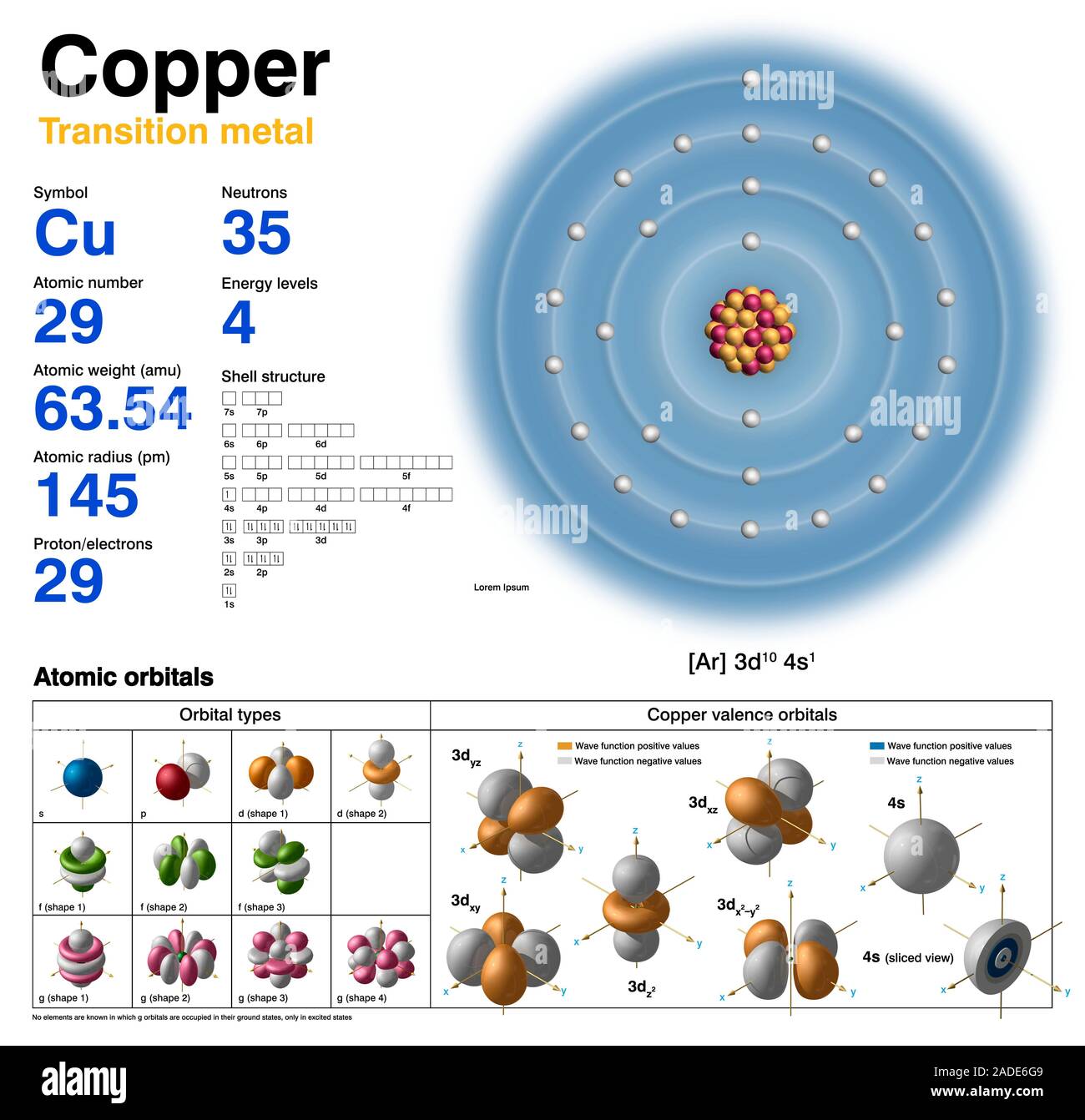
Copper Electron Configuration In Shells . the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. electronic configuration of cu. The only difference is at the end of the configuration that is in the 3d and 4s shells. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The electronic configuration of copper (cu) can be represented as: copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of.
from www.alamy.com
in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. electronic configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The only difference is at the end of the configuration that is in the 3d and 4s shells. The electronic configuration of copper (cu) can be represented as:
Copper (Cu). Diagram of the nuclear composition, electron configuration
Copper Electron Configuration In Shells electronic configuration of cu. electronic configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. The electronic configuration of copper (cu) can be represented as: copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration In Shells electronic configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The only difference is at the end of the configuration that is in the 3d and 4s shells. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. the electron. Copper Electron Configuration In Shells.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The only difference is at the end of the configuration that is in the 3d and 4s shells. electronic configuration of cu. The electronic configuration of copper (cu) can be. Copper Electron Configuration In Shells.
From www.youtube.com
Electronic Configuration of Chromium and Copper Structure of Atom Copper Electron Configuration In Shells If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. electronic configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The only difference is at the end of the configuration that. Copper Electron Configuration In Shells.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electron Configuration In Shells copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration. Copper Electron Configuration In Shells.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper Electron Configuration In Shells 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s. Copper Electron Configuration In Shells.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electron Configuration In Shells The only difference is at the end of the configuration that is in the 3d and 4s shells. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the. Copper Electron Configuration In Shells.
From donghokiddy.com
Does Copper Really Have 3 Valence Electrons? Exploring Its Atomic Copper Electron Configuration In Shells The electronic configuration of copper (cu) can be represented as: copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The only difference is. Copper Electron Configuration In Shells.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Configuration In Shells The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. If the general pattern. Copper Electron Configuration In Shells.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration In Shells the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. copper is a chemical element of the periodic table with chemical symbol cu and atomic. Copper Electron Configuration In Shells.
From en-academic.com
Copper Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The electronic configuration of copper (cu) can be represented as: 1s 2 2s 2 2p 6 3 s 2 3p 6. Copper Electron Configuration In Shells.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The electronic configuration of copper (cu) can be represented as: electronic configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. If the general pattern of filling electron orbitals. Copper Electron Configuration In Shells.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electron Configuration In Shells how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The electronic configuration of copper (cu) can be represented as: copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. 1s2 2s2 2p6. Copper Electron Configuration In Shells.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. the electron distribution in. Copper Electron Configuration In Shells.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Copper Electron Configuration In Shells If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The electronic configuration of copper (cu) can be represented as: electronic configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. . Copper Electron Configuration In Shells.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration In Shells If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. in. Copper Electron Configuration In Shells.
From www.schoolmykids.com
Compare Copper vs Carbon Periodic Table Element Comparison Compare Copper Electron Configuration In Shells in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The only difference is at. Copper Electron Configuration In Shells.
From pnghut.com
Bohr Model Electron Shell Copper Atom Valence Lewis Structure Copper Electron Configuration In Shells in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper is a chemical element of the periodic table with chemical symbol cu and atomic. Copper Electron Configuration In Shells.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration In Shells The only difference is at the end of the configuration that is in the 3d and 4s shells. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. electronic configuration of cu. in this article, we will explore the electron orbital arrangement, shell configuration, and. Copper Electron Configuration In Shells.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Configuration In Shells 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. how to. Copper Electron Configuration In Shells.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electron Configuration In Shells how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron. Copper Electron Configuration In Shells.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Copper Electron Configuration In Shells The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. how to write the electron configuration for copper (cu, cu+, and cu2+). Copper Electron Configuration In Shells.
From sciencenotes.org
List of Electron Configurations of Elements Copper Electron Configuration In Shells copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. If the general pattern of filling electron orbitals is followed, then copper’s. Copper Electron Configuration In Shells.
From wiringguidefrosts.z19.web.core.windows.net
Copper Electron Configuration Diagram Copper Electron Configuration In Shells how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. copper. Copper Electron Configuration In Shells.
From www.slideshare.net
Copper Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. 1s 2. Copper Electron Configuration In Shells.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Electron Configuration In Shells the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. how. Copper Electron Configuration In Shells.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Electron Configuration In Shells copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The only difference is at the end of. Copper Electron Configuration In Shells.
From chemaddicts.blogspot.com
Chemaddicts The interpreting electronic structure in box notation Copper Electron Configuration In Shells 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d. Copper Electron Configuration In Shells.
From www.animalia-life.club
Electron Shell Diagram Copper Electron Configuration In Shells how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The only difference is at the end of the configuration that is in the 3d and 4s shells. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located. Copper Electron Configuration In Shells.
From izayahmeowcrosby.blogspot.com
Electronic Configuration of Copper Copper Electron Configuration In Shells how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The. Copper Electron Configuration In Shells.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration In Shells The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+). Copper Electron Configuration In Shells.
From www.youtube.com
How to write/find/do the electron configuration of Cr(Chromium) and Cu Copper Electron Configuration In Shells The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. copper is a chemical element. Copper Electron Configuration In Shells.
From www.animalia-life.club
Electron Shell Diagram Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s 2 2s 2. Copper Electron Configuration In Shells.
From www.webelements.com
Elements Periodic Table » Copper » properties of free atoms Copper Electron Configuration In Shells in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. The electronic configuration of copper (cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Copper Electron Configuration In Shells.
From www.alamy.com
Symbol and electron diagram for Copper Stock Vector Image & Art Alamy Copper Electron Configuration In Shells If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. in this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper. The only difference is at the end of the configuration that is in the 3d and 4s shells. 1s2. Copper Electron Configuration In Shells.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Electron Configuration In Shells 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration. Copper Electron Configuration In Shells.