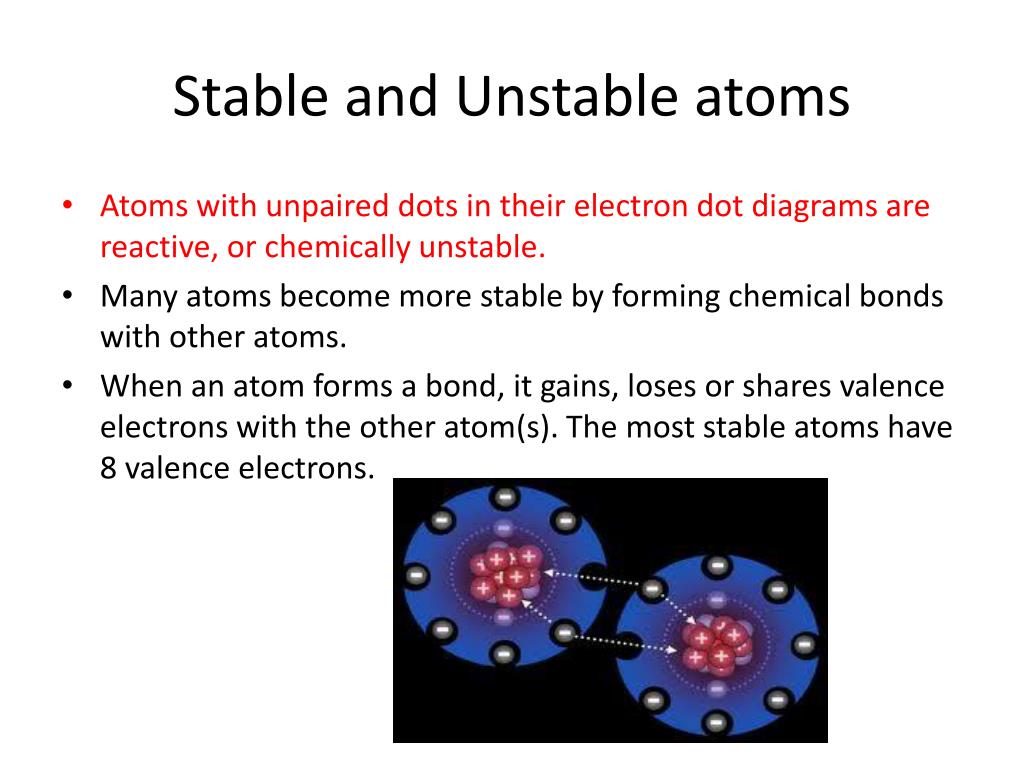
Why Is Hydrogen Stable . hydrogen can be both stable and unstable at the same time, depending on several key factors. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Hydrogen is unstable in its natural. why does a hydrogen bond occur?
from www.slideserve.com
Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. why does a hydrogen bond occur? Hydrogen is unstable in its natural. hydrogen can be both stable and unstable at the same time, depending on several key factors. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1.
PPT Chapter 8 lesson 1 Electrons and energy levels PowerPoint
Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen can be both stable and unstable at the same time, depending on several key factors. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. why does a hydrogen bond occur? Hydrogen is unstable in its natural.
From www.bbc.co.uk
What is hydrogen guide for KS3 chemistry students BBC Bitesize Why Is Hydrogen Stable why does a hydrogen bond occur? Hydrogen is unstable in its natural. hydrogen can be both stable and unstable at the same time, depending on several key factors. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. Since the hydrogen donor (n, o, or f). Why Is Hydrogen Stable.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Why Is Hydrogen Stable why does a hydrogen bond occur? hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. Hydrogen is unstable in its natural. hydrogen can be both stable and unstable at the same time, depending on several key factors. \(h _2 o\) is the most abundant. Why Is Hydrogen Stable.
From www.youtube.com
Isotopes of hydrogen YouTube Why Is Hydrogen Stable Hydrogen is unstable in its natural. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. because a hydrogen. Why Is Hydrogen Stable.
From www.researchgate.net
A Stable hydrogen and oxygen isotope distribution of water in Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen can be both stable and unstable at the same time, depending on several key factors. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation. Why Is Hydrogen Stable.
From hebasoffar.blogspot.com
Science online The electronic configuration and the chemical activity Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. Hydrogen is unstable in its natural. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. why does a hydrogen bond occur? \(h _2 o\) is the most. Why Is Hydrogen Stable.
From sayngon.com
Are Hydrogen Bonds The Strongest Interactions Between Molecules? Why Is Hydrogen Stable Hydrogen is unstable in its natural. hydrogen can be both stable and unstable at the same time, depending on several key factors. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. why does a hydrogen bond occur? Since the. Why Is Hydrogen Stable.
From www.researchgate.net
(a) Stable isotope composition of hydrogen (δ 2 H) and oxygen (δ 18 O Why Is Hydrogen Stable \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. Hydrogen is unstable in its natural. Since the. Why Is Hydrogen Stable.
From www.examples.com
Hydrogen (H) Definition, Preparation, Properties, Uses, Compounds Why Is Hydrogen Stable \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Hydrogen is unstable in its natural. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen. Why Is Hydrogen Stable.
From socratic.org
Two hydrogen atoms interact to form a hydrogen molecule. Classify the Why Is Hydrogen Stable Hydrogen is unstable in its natural. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. why does a hydrogen bond occur? because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is. Why Is Hydrogen Stable.
From modernhydrogen.com
Distributed Hydrogen Production Why It's The Future Of Clean Energy Why Is Hydrogen Stable hydrogen can be both stable and unstable at the same time, depending on several key factors. why does a hydrogen bond occur? Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try. Why Is Hydrogen Stable.
From www.geeksforgeeks.org
Isotopes Definition, Examples, Types, Applications and FAQs Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. Hydrogen is unstable in its natural. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. hydrogen is oxidized by elements that are more electronegative to form compounds in. Why Is Hydrogen Stable.
From www.researchgate.net
Most stable hydrogen adsorption structures on Pd, In, and PdIn alloy Why Is Hydrogen Stable hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. hydrogen can be both stable and unstable at the. Why Is Hydrogen Stable.
From flipboard.com
New approach to fabricating ionconducting ceramic membranes for stable Why Is Hydrogen Stable Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. Hydrogen is unstable in its natural. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. because a hydrogen atom with its one electron in this orbit has the lowest possible. Why Is Hydrogen Stable.
From blog.aramsco.com
Hydrogen Peroxide Stability Why Is Hydrogen Stable why does a hydrogen bond occur? hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. hydrogen can. Why Is Hydrogen Stable.
From www.researchgate.net
(a) Schematic illustration of stable hydrogen production using Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. why does a hydrogen bond occur? . Why Is Hydrogen Stable.
From www.slideserve.com
PPT Hydrogen Bonding PowerPoint Presentation, free download ID297441 Why Is Hydrogen Stable hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. hydrogen can be both stable and unstable at the same time, depending on several key factors. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground. Why Is Hydrogen Stable.
From sayngon.com
Are Hydrogen Bonds Weak Or Strong? Exploring Their True Nature Why Is Hydrogen Stable \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Hydrogen is unstable in its natural. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. why does a. Why Is Hydrogen Stable.
From atlantichydrogen.ca
Why Hydrogen? Atlantic Hydrogen Alliance Why Is Hydrogen Stable \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Hydrogen is unstable in its natural. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen. Why Is Hydrogen Stable.
From www.slideserve.com
PPT Chapter 8 lesson 1 Electrons and energy levels PowerPoint Why Is Hydrogen Stable why does a hydrogen bond occur? hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Since the hydrogen. Why Is Hydrogen Stable.
From www.researchgate.net
Summary of relation between stable hydrogen absorption rate (iH) and an Why Is Hydrogen Stable Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. why does a hydrogen bond occur? \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. because a hydrogen atom with its one electron. Why Is Hydrogen Stable.
From www.researchgate.net
Selected nonmetals and their interaction with hydrogen to form stable Why Is Hydrogen Stable hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Since the hydrogen donor (n, o, or f) is strongly. Why Is Hydrogen Stable.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii Why Is Hydrogen Stable Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. Hydrogen is unstable in its natural. hydrogen can be both stable and unstable at the same time, depending on several key factors. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of. Why Is Hydrogen Stable.
From www.vrogue.co
Formation Of Hydrogen Bonds In Water Easybiologyclass vrogue.co Why Is Hydrogen Stable Hydrogen is unstable in its natural. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. why does a hydrogen bond occur? hydrogen can. Why Is Hydrogen Stable.
From www.thechemicalengineer.com
Why Hydrogen? Features The Chemical Engineer Why Is Hydrogen Stable \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Hydrogen is unstable in its natural. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. why does a. Why Is Hydrogen Stable.
From julien-has-bryant.blogspot.com
Why Are Hydrogen Bonds Stronger Than Dipole Dipole JulienhasBryant Why Is Hydrogen Stable Hydrogen is unstable in its natural. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. hydrogen can be both stable and unstable. Why Is Hydrogen Stable.
From www.weforum.org
Why hydrogen could be the future of green energy World Economic Forum Why Is Hydrogen Stable hydrogen can be both stable and unstable at the same time, depending on several key factors. why does a hydrogen bond occur? because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. \(h _2 o\) is the most abundant form of hydrogen on. Why Is Hydrogen Stable.
From www.masterorganicchemistry.com
Alkene Stability Increases With Substitution Master Organic Chemistry Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. Hydrogen is unstable in its natural. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. hydrogen is oxidized by elements that are more electronegative to form compounds in. Why Is Hydrogen Stable.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. why does a hydrogen bond occur? hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most. Why Is Hydrogen Stable.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Why Is Hydrogen Stable Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. Hydrogen is unstable in its natural. why does a hydrogen bond occur? because a hydrogen atom with its one electron. Why Is Hydrogen Stable.
From pubs.acs.org
Hydrogen Stability and Bonding in SiNx and Al2O3 Dielectric Stacks on Why Is Hydrogen Stable hydrogen can be both stable and unstable at the same time, depending on several key factors. because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. why does. Why Is Hydrogen Stable.
From www.researchgate.net
Stable hydrogen and oxygen isotope composition of groundwater samples Why Is Hydrogen Stable \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract hydrogen from water without electrolysis of. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. Hydrogen is unstable in its natural. hydrogen can be both stable and unstable at the. Why Is Hydrogen Stable.
From ar.inspiredpencil.com
Carbon Hydrogen Covalent Bond Why Is Hydrogen Stable because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most. hydrogen can be both stable and unstable at the same time, depending on several key factors. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation. Why Is Hydrogen Stable.
From www.myxxgirl.com
Types Of Hydrogen Isotopes Chemistry Basics Chemistry Science Chemistry Why Is Hydrogen Stable why does a hydrogen bond occur? hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. hydrogen can be both stable and unstable at the same time, depending on several. Why Is Hydrogen Stable.
From www.chemistrysteps.com
Alkenes Structure and Stability Chemistry Steps Why Is Hydrogen Stable hydrogen can be both stable and unstable at the same time, depending on several key factors. why does a hydrogen bond occur? hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. because a hydrogen atom with its one electron in this orbit has the. Why Is Hydrogen Stable.
From xecogioinhapkhau.com
Why Does Carbon Stable Unveiling Its Atomic Secrets Why Is Hydrogen Stable Since the hydrogen donor (n, o, or f) is strongly electronegative, it pulls the covalently bonded. hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1. \(h _2 o\) is the most abundant form of hydrogen on the planet, so it seems logical to try to extract. Why Is Hydrogen Stable.