Catalyst In Endothermic Reaction . Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. It covers changes to the. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Describe three ways to speed up a reaction. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. We have two different catalysts that speed up the decomposition of. A catalyst is a substance that speeds up the rate of a chemical reaction. Unfortunately, for many reactions, the real shapes of the energy. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature.
from www.numerade.com
Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. We have two different catalysts that speed up the decomposition of. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Describe three ways to speed up a reaction. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. A catalyst is a substance that speeds up the rate of a chemical reaction. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Unfortunately, for many reactions, the real shapes of the energy. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.
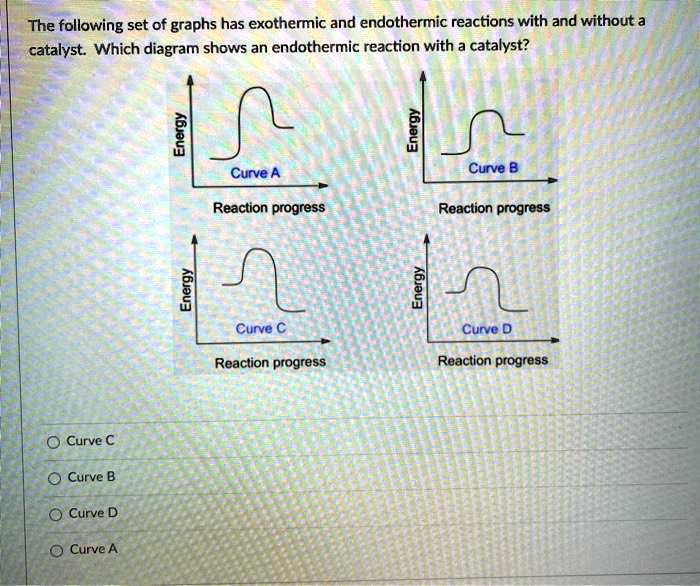
SOLVED The following set of graphs has exothermic and endothermic
Catalyst In Endothermic Reaction Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. A catalyst is a substance that speeds up the rate of a chemical reaction. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Describe three ways to speed up a reaction. Unfortunately, for many reactions, the real shapes of the energy. We have two different catalysts that speed up the decomposition of. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. It covers changes to the. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Catalyst In Endothermic Reaction Unfortunately, for many reactions, the real shapes of the energy. It covers changes to the. A catalyst is a substance that speeds up the rate of a chemical reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to. Catalyst In Endothermic Reaction.
From nonsibihighschool.org
Section 184 Catalysis Catalyst In Endothermic Reaction This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. It covers changes to the. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.. Catalyst In Endothermic Reaction.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst In Endothermic Reaction A catalyst is a substance that speeds up the rate of a chemical reaction. Unfortunately, for many reactions, the real shapes of the energy. We have two different catalysts that speed up the decomposition of. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. For an endothermic chemical reaction to proceed, the reactants must absorb. Catalyst In Endothermic Reaction.
From slideplayer.com
Catalysts, Endothermic vs. Exothermic Reactions ppt download Catalyst In Endothermic Reaction A catalyst is a substance that speeds up the rate of a chemical reaction. We have two different catalysts that speed up the decomposition of. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. It covers changes to the. This page looks at le chatelier's principle and explains how to apply it to reactions in. Catalyst In Endothermic Reaction.
From www.teachoo.com
Chemical Equation Meaning, How to Write [with 5+ Examples] Teachoo Catalyst In Endothermic Reaction Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. It covers changes. Catalyst In Endothermic Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst In Endothermic Reaction Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. We have two different catalysts that speed up the decomposition of. Unfortunately, for many reactions, the real shapes of the energy. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. It covers changes to. Catalyst In Endothermic Reaction.
From www.revisechemistry.uk
Energetics OCR Gateway C3 revisechemistry.uk Catalyst In Endothermic Reaction Describe three ways to speed up a reaction. A catalyst is a substance that speeds up the rate of a chemical reaction. Unfortunately, for many reactions, the real shapes of the energy. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Exothermic reactions transfer energy to the surroundings. Catalyst In Endothermic Reaction.
From www.sliderbase.com
Endothermic Reaction w/Catalyst Catalyst In Endothermic Reaction Describe three ways to speed up a reaction. We have two different catalysts that speed up the decomposition of. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.. Catalyst In Endothermic Reaction.
From www.sliderbase.com
Endothermic Reaction w/Catalyst Catalyst In Endothermic Reaction For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Describe three ways to speed up a reaction. Unfortunately, for many reactions, the real shapes of the energy. It covers changes to the. A catalyst is a substance that speeds up the rate of a chemical reaction. Exothermic reactions transfer. Catalyst In Endothermic Reaction.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst In Endothermic Reaction Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Describe three ways to speed up a reaction. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. It covers changes to the. This page looks at le chatelier's principle and explains how to apply it. Catalyst In Endothermic Reaction.
From usermanualwhelming.z21.web.core.windows.net
Reaction Profile Diagram With Catalyst Catalyst In Endothermic Reaction It covers changes to the. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. This page looks at le chatelier's. Catalyst In Endothermic Reaction.
From slideplayer.com
Catalysts, Endothermic vs. Exothermic Reactions ppt download Catalyst In Endothermic Reaction Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. We have two different catalysts that speed up the decomposition of.. Catalyst In Endothermic Reaction.
From www.slideserve.com
PPT Chapter 18 “Reaction Rates and Equilibrium” PowerPoint Catalyst In Endothermic Reaction Describe three ways to speed up a reaction. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy. Catalyst In Endothermic Reaction.
From slideplayer.com
Catalysts, Endothermic vs. Exothermic Reactions ppt download Catalyst In Endothermic Reaction This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. It covers changes to the. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature.. Catalyst In Endothermic Reaction.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID3558810 Catalyst In Endothermic Reaction We have two different catalysts that speed up the decomposition of. It covers changes to the. Unfortunately, for many reactions, the real shapes of the energy. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a. Catalyst In Endothermic Reaction.
From www.numerade.com
SOLVED The following set of graphs has exothermic and endothermic Catalyst In Endothermic Reaction It covers changes to the. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. We have two different catalysts that speed up the decomposition of.. Catalyst In Endothermic Reaction.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalyst In Endothermic Reaction Unfortunately, for many reactions, the real shapes of the energy. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. We have two different catalysts that speed up the decomposition of. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. A catalyst is. Catalyst In Endothermic Reaction.
From slideplayer.com
Catalysts, Endothermic vs. Exothermic Reactions ppt download Catalyst In Endothermic Reaction Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. It covers changes to the. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Differentiate. Catalyst In Endothermic Reaction.
From www.slideserve.com
PPT IQ 1 PowerPoint Presentation, free download ID3580948 Catalyst In Endothermic Reaction This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. Catalyst In Endothermic Reaction.
From evulpo.com
Exothermic and endothermic reactions and catalysts Science Catalyst In Endothermic Reaction This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Differentiate between reversible exothermic, irreversible. Catalyst In Endothermic Reaction.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Catalyst In Endothermic Reaction A catalyst is a substance that speeds up the rate of a chemical reaction. Describe three ways to speed up a reaction. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Unfortunately, for many reactions, the real shapes of the energy. For an endothermic chemical reaction to proceed, the. Catalyst In Endothermic Reaction.
From ar.inspiredpencil.com
Endothermic Reaction With Catalyst Catalyst In Endothermic Reaction Describe three ways to speed up a reaction. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy. Catalyst In Endothermic Reaction.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Catalyst In Endothermic Reaction We have two different catalysts that speed up the decomposition of. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. This page looks at le chatelier's principle and explains how to apply it to. Catalyst In Endothermic Reaction.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Catalyst In Endothermic Reaction Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. We have two different catalysts that speed up the decomposition of. Describe three ways to speed up a reaction. This page looks at le chatelier's. Catalyst In Endothermic Reaction.
From www.slideserve.com
PPT Chemical Rxn Rates PowerPoint Presentation, free download ID Catalyst In Endothermic Reaction Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products. A catalyst is a substance that speeds up the rate of a chemical reaction. Differentiate between reversible exothermic, irreversible exothermic,. Catalyst In Endothermic Reaction.
From 2012books.lardbucket.org
Catalysis Catalyst In Endothermic Reaction Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. A catalyst is a substance that speeds up the rate of a chemical reaction. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Describe three ways to speed up. Catalyst In Endothermic Reaction.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Catalyst In Endothermic Reaction Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. We have two different catalysts. Catalyst In Endothermic Reaction.
From nonsibihighschool.org
181) Catalyst In Endothermic Reaction We have two different catalysts that speed up the decomposition of. It covers changes to the. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Exothermic reactions in solution give out energy and the. Catalyst In Endothermic Reaction.
From slideplayer.com
Catalysts, Endothermic vs. Exothermic Reactions ppt download Catalyst In Endothermic Reaction Unfortunately, for many reactions, the real shapes of the energy. It covers changes to the. Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. For an endothermic chemical reaction to proceed, the reactants must absorb. Catalyst In Endothermic Reaction.
From ar.inspiredpencil.com
Endothermic Reaction With Catalyst Catalyst In Endothermic Reaction Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. A catalyst is a substance that speeds up the rate of a chemical reaction. Unfortunately, for many reactions, the real shapes of the energy. We. Catalyst In Endothermic Reaction.
From studylib.net
Lesson 7 Reaction profiles Catalyst In Endothermic Reaction We have two different catalysts that speed up the decomposition of. A catalyst is a substance that speeds up the rate of a chemical reaction. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and. Catalyst In Endothermic Reaction.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Catalyst In Endothermic Reaction A catalyst is a substance that speeds up the rate of a chemical reaction. Unfortunately, for many reactions, the real shapes of the energy. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in energy and the temperature. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue. Catalyst In Endothermic Reaction.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst In Endothermic Reaction Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Unfortunately, for many reactions, the real shapes of the energy. Describe three ways to speed up a reaction. We have two different catalysts that speed up the decomposition of. For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be. Catalyst In Endothermic Reaction.
From byjus.com
24. What is the Minimum activation energy required for endothermic and Catalyst In Endothermic Reaction Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. A catalyst is a substance that speeds up the rate of a chemical reaction. Differentiate between reversible exothermic, irreversible. Catalyst In Endothermic Reaction.
From manualpartsynaxis123.z13.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Catalyst In Endothermic Reaction It covers changes to the. Describe three ways to speed up a reaction. Figure 12.19 reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. We have two different catalysts that speed up the decomposition of. This page looks at le chatelier's principle and explains how to apply it to reactions in. Catalyst In Endothermic Reaction.