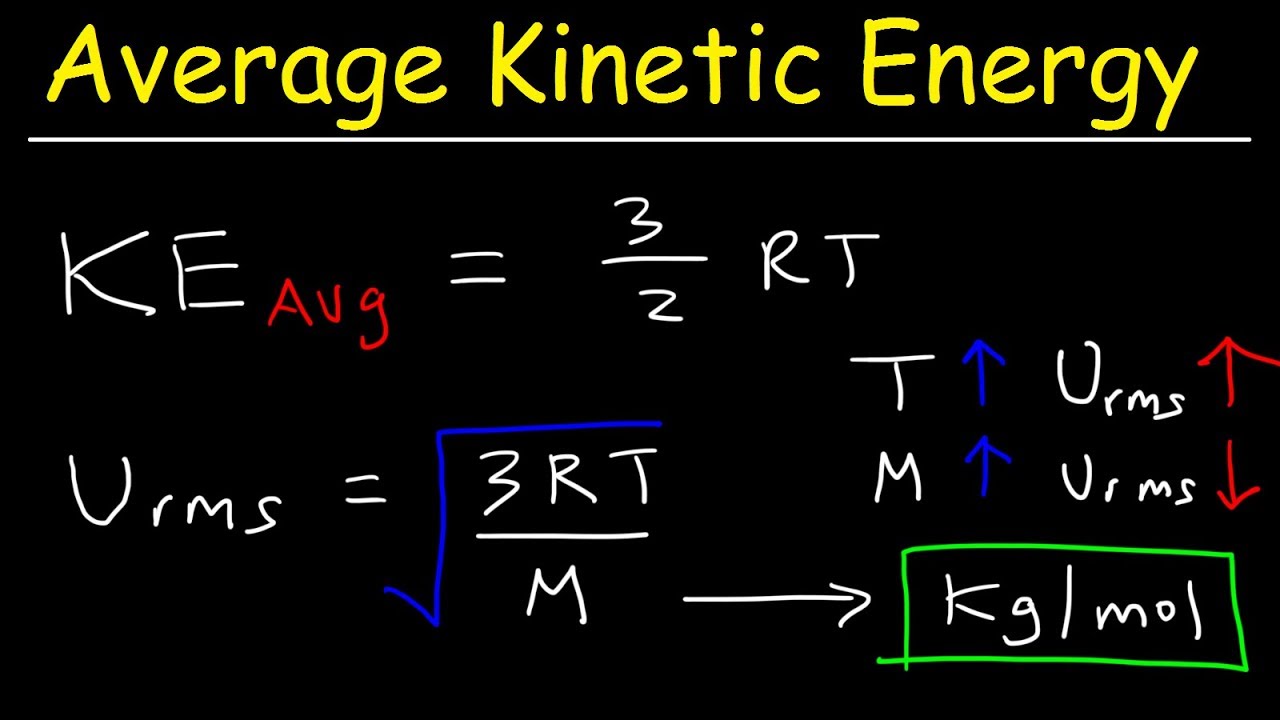
Gas Law Kinetic Energy . the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. The test of the kmt and its postulates is its ability to. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. In this video, we'll see how. How is the rms speed different from the. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. Equipartition of energy and the ideal gas law 29.1. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic.
from www.youtube.com
what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? How is the rms speed different from the. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. Equipartition of energy and the ideal gas law 29.1. The test of the kmt and its postulates is its ability to. In this video, we'll see how.
Average Energy of a Gas and Root Mean Square Velocity Practice
Gas Law Kinetic Energy In this video, we'll see how. The test of the kmt and its postulates is its ability to. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. How is the rms speed different from the. Equipartition of energy and the ideal gas law 29.1. In this video, we'll see how. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases.
From socratic.org
The theory assumes that collisions of gas particles are Gas Law Kinetic Energy the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. what happens to the average kinetic energy of a gas if the rms speed of its. Gas Law Kinetic Energy.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? The test of the kmt and its postulates is its ability to. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of. Gas Law Kinetic Energy.
From selenarosjohns.blogspot.com
Molecular Theory of Gases SelenarosJohns Gas Law Kinetic Energy In this video, we'll see how. The test of the kmt and its postulates is its ability to. Equipartition of energy and the ideal gas law 29.1. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the kinetic molecular theory (kmt) can be used to explain. Gas Law Kinetic Energy.
From www.youtube.com
The Average Energy per Molecule Equation for an Ideal Gas IB Gas Law Kinetic Energy The test of the kmt and its postulates is its ability to. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. according to the kinetic molecular. Gas Law Kinetic Energy.
From manualworshipped.z14.web.core.windows.net
What Does Boyle's Law Describe Gas Law Kinetic Energy what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the ideal gas law can be expressed in terms of the mass of. Gas Law Kinetic Energy.
From ecampusontario.pressbooks.pub
2.6 Theory of Gases (Ideal Gas Behaviours Gas Law Kinetic Energy the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. The test of the kmt and its postulates is its ability to. the average kinetic energy of the gas molecules. Gas Law Kinetic Energy.
From www.studiestoday.com
NEET UG Physics Theory of Gases MCQs, Multiple Choice Questions Gas Law Kinetic Energy the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. In this video, we'll see how. Equipartition of energy and the ideal gas law 29.1. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of. Gas Law Kinetic Energy.
From www.slideserve.com
PPT Physics 101 Lecture 29 Ideal Gas Law & Theory PowerPoint Gas Law Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. The test of the kmt and its postulates is its ability to. How is the rms speed different from the. Equipartition of energy and the. Gas Law Kinetic Energy.
From www.youtube.com
Derivation of of Gas Equation YouTube Gas Law Kinetic Energy the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. In this video, we'll see how. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the ideal gas law can be expressed. Gas Law Kinetic Energy.
From www.youtube.com
Average Energy of a Gas and Root Mean Square Velocity Practice Gas Law Kinetic Energy the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. Equipartition of energy and the ideal gas law 29.1. How is the rms speed different from the. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. The test of. Gas Law Kinetic Energy.
From www.sliderbase.com
Gas Laws Presentation Chemistry Gas Law Kinetic Energy what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? In this video, we'll see how. Equipartition of energy and the ideal gas law 29.1. How is the rms speed different from the. the ideal gas law can be expressed in terms of the mass. Gas Law Kinetic Energy.
From brainly.in
Graham's law of diffusion from gas equation Brainly.in Gas Law Kinetic Energy The test of the kmt and its postulates is its ability to. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the average kinetic energy of the gas molecules. Gas Law Kinetic Energy.
From glancychem.com
Chemistry Resources 3 GlancyChem Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. In this video, we'll see how. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kinetic molecular theory (kmt). Gas Law Kinetic Energy.
From psiberg.com
Molecular Theory of Gases Postulates, and Gas Laws PSIBERG Gas Law Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. In this video, we'll see how. How is the rms speed different from the. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. what happens to the average. Gas Law Kinetic Energy.
From saylordotorg.github.io
Theory of Gases Gas Law Kinetic Energy How is the rms speed different from the. In this video, we'll see how. The test of the kmt and its postulates is its ability to. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the kinetic molecular theory (kmt) can be used to explain the. Gas Law Kinetic Energy.
From www.youtube.com
8.17 How do i derive the theory of gas equation? YouTube Gas Law Kinetic Energy according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is. Gas Law Kinetic Energy.
From www.grc.nasa.gov
Theory of Gases Gas Law Kinetic Energy How is the rms speed different from the. Equipartition of energy and the ideal gas law 29.1. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? In. Gas Law Kinetic Energy.
From www.youtube.com
theory of Gases Assumption Introduction Derivation of Gas Law Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. How is the rms speed different from the. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. what happens to the average kinetic energy of a gas if the. Gas Law Kinetic Energy.
From www.priyamstudycentre.com
Theory of Gases Gas Equation, Postulates, Formula Gas Law Kinetic Energy How is the rms speed different from the. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. In this video, we'll see how. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. what happens to. Gas Law Kinetic Energy.
From www.slideshare.net
theory of gases Gas Law Kinetic Energy according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? The test of the kmt and its postulates is its ability to. the average. Gas Law Kinetic Energy.
From beaucelatkinson.blogspot.com
Molecular Theory of Gases BeaucelAtkinson Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. How is the rms speed different from the. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average. Gas Law Kinetic Energy.
From www.youtube.com
Molecular Theory & Ideal Gas Law Derivation YouTube Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. The test of the kmt and its postulates. Gas Law Kinetic Energy.
From owlcation.com
The Theories and Behavior of Gas Owlcation Gas Law Kinetic Energy what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. The test of the kmt and its postulates is its ability to.. Gas Law Kinetic Energy.
From www.sliderbase.com
Molecular Theory Presentation Chemistry Gas Law Kinetic Energy The test of the kmt and its postulates is its ability to. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. the ideal gas law can be expressed in terms of the mass. Gas Law Kinetic Energy.
From www.slideserve.com
PPT The Ideal Gas Laws PowerPoint Presentation, free download ID Gas Law Kinetic Energy the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the ideal gas law can be expressed in terms of the mass of the gas’s molecules and. Gas Law Kinetic Energy.
From www.researchgate.net
(PDF) Worked Examples on Gas Laws and Theory Gas Law Kinetic Energy according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute. The test of the kmt and its postulates is its ability to. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the average. Gas Law Kinetic Energy.
From www.slideserve.com
PPT Theory of Gases PowerPoint Presentation, free download Gas Law Kinetic Energy the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. How is the rms speed different from the. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kinetic molecular theory of gases (kmt or simply. Gas Law Kinetic Energy.
From www.slideserve.com
PPT The Ideal Gas Laws PowerPoint Presentation, free download ID Gas Law Kinetic Energy How is the rms speed different from the. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. Equipartition of energy and the ideal gas law 29.1. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that. Gas Law Kinetic Energy.
From www.youtube.com
Theory of gases Lecture 2 Derivation of Gas Equation Gas Law Kinetic Energy the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. Equipartition of energy and the ideal gas law 29.1. How is the rms speed different from the. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. In this. Gas Law Kinetic Energy.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint Gas Law Kinetic Energy the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? the kinetic molecular theory (kmt) can be used to explain the. Gas Law Kinetic Energy.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is. Gas Law Kinetic Energy.
From www.toppr.com
37 (a)On the basis of theory of gases derive an expression the Gas Law Kinetic Energy The test of the kmt and its postulates is its ability to. Equipartition of energy and the ideal gas law 29.1. the average kinetic energy of the gas molecules is proportional to the kelvin temperature of the gas. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a. Gas Law Kinetic Energy.
From www.youtube.com
Equation Of Gases YouTube Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. the ideal gas law can be expressed in terms of the mass of the gas’s molecules and \(\bar{v^2}\), the average of the molecular. the kinetic molecular theory (kmt) can be used to explain the macroscopic behavior of ideal gases. according to the kinetic molecular theory, the average kinetic. Gas Law Kinetic Energy.
From www.slideserve.com
PPT Lecture 8 The Gas Laws. Theory of Matter. PowerPoint Gas Law Kinetic Energy The test of the kmt and its postulates is its ability to. the kinetic molecular theory of gases (kmt or simply kinetic theory of gases) is a theoretical model that explains the macroscopic. In this video, we'll see how. How is the rms speed different from the. Equipartition of energy and the ideal gas law 29.1. the average. Gas Law Kinetic Energy.
From www.youtube.com
Gas Laws Intro YouTube Gas Law Kinetic Energy Equipartition of energy and the ideal gas law 29.1. In this video, we'll see how. what happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute.. Gas Law Kinetic Energy.