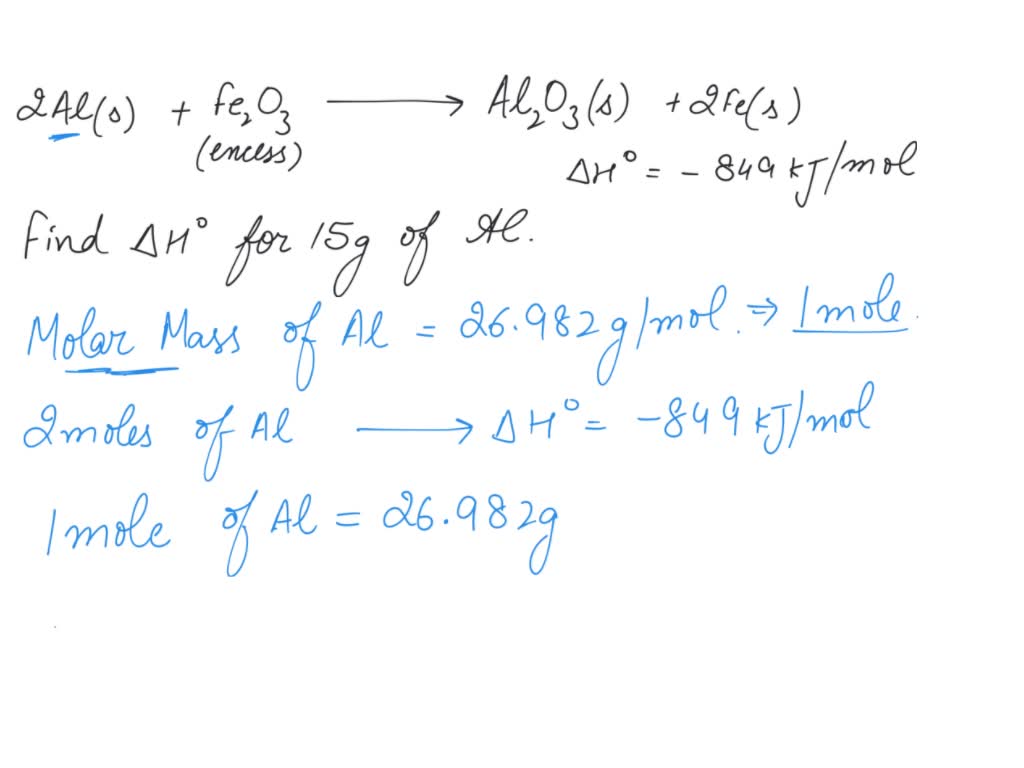Iron Iii Oxide Reacts With Aluminum . This shows that aluminium is above iron in the reactivity series. Reactions between metals and metal. Given below is the chemical. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Mohammad razak of the city college of new york's chemistry department shows us a. Aluminum replaces the metal in the oxide. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. In the reaction between aluminum and iron(iii) oxide, this forms iron and. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. This is because aluminum is more reactive than iron. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). This mixture of aluminum and iron oxide is called. The aluminium removes oxygen from the iron (iii) oxide: Iron(iii) oxide + aluminium → aluminium oxide + iron.
from www.numerade.com
This shows that aluminium is above iron in the reactivity series. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). The aluminium removes oxygen from the iron (iii) oxide: Given below is the chemical. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminum replaces the metal in the oxide. This is because aluminum is more reactive than iron. Mohammad razak of the city college of new york's chemistry department shows us a. In the reaction between aluminum and iron(iii) oxide, this forms iron and.
SOLVED Determine the heat evolved (in kJ) when 15.0 g of Aluminum
Iron Iii Oxide Reacts With Aluminum Aluminum replaces the metal in the oxide. Aluminum replaces the metal in the oxide. Reactions between metals and metal. This shows that aluminium is above iron in the reactivity series. Mohammad razak of the city college of new york's chemistry department shows us a. In the reaction between aluminum and iron(iii) oxide, this forms iron and. Iron(iii) oxide + aluminium → aluminium oxide + iron. This is because aluminum is more reactive than iron. This mixture of aluminum and iron oxide is called. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. The aluminium removes oxygen from the iron (iii) oxide: Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Given below is the chemical. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c).
From www.chegg.com
Solved 3. Given that iron(III) oxide reacts with aluminum Iron Iii Oxide Reacts With Aluminum The aluminium removes oxygen from the iron (iii) oxide: This shows that aluminium is above iron in the reactivity series. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Given below is the chemical. Iron (iii) oxide reacts with aluminium and gives molten. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVEDIn the thermite reaction, iron(III) oxide is reduced by aluminum Iron Iii Oxide Reacts With Aluminum Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Given below is the chemical. This shows that aluminium is above iron in the reactivity series. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe).. Iron Iii Oxide Reacts With Aluminum.
From www.chegg.com
Solved The reaction between aluminum and iron(III) oxide Iron Iii Oxide Reacts With Aluminum Given below is the chemical. Aluminum replaces the metal in the oxide. This shows that aluminium is above iron in the reactivity series. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This is because aluminum is more reactive than iron. Iron(iii) oxide + aluminium. Iron Iii Oxide Reacts With Aluminum.
From www.gauthmath.com
Solved 6 3. At high temperatures iron(III) oxide reacts with Iron Iii Oxide Reacts With Aluminum Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Given below is the chemical. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This mixture of aluminum and. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVEDThe formation of rust [Iron (III) oxide] on the surface of iron Iron Iii Oxide Reacts With Aluminum This mixture of aluminum and iron oxide is called. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. This shows that aluminium is above iron in the reactivity series. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This is because. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVEDThe reaction of iron(III) oxide with aluminum to give molten Iron Iii Oxide Reacts With Aluminum Iron(iii) oxide + aluminium → aluminium oxide + iron. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3). Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED If 60.0 g of molten iron(III) oxide reacts with 31.5 g of Iron Iii Oxide Reacts With Aluminum Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. The aluminium removes. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVEDIf 37.5 g of molten iron(III) oxide reacts with 175 g of Iron Iii Oxide Reacts With Aluminum Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. In the reaction between aluminum and iron(iii) oxide, this forms iron and. This mixture of aluminum and iron oxide is called. Given below is the chemical. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting. Iron Iii Oxide Reacts With Aluminum.
From www.alamy.com
A thermite reaction (iron (III) oxide & aluminium powder) in a UK Iron Iii Oxide Reacts With Aluminum This mixture of aluminum and iron oxide is called. Iron(iii) oxide + aluminium → aluminium oxide + iron. Reactions between metals and metal. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Given below is the chemical. Iron (iii) oxide reacts with aluminium. Iron Iii Oxide Reacts With Aluminum.
From www.youtube.com
Thermit reaction, iron (III) oxide reacts with aluminium andgives Iron Iii Oxide Reacts With Aluminum This is because aluminum is more reactive than iron. In the reaction between aluminum and iron(iii) oxide, this forms iron and. This mixture of aluminum and iron oxide is called. Aluminum replaces the metal in the oxide. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Mohammad razak of the city college of new york's chemistry. Iron Iii Oxide Reacts With Aluminum.
From www.chegg.com
Solved When iron(III) oxide reacts with aluminum, aluminum Iron Iii Oxide Reacts With Aluminum A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This is because aluminum is more reactive than iron. Reactions between metals and metal. Aluminum replaces the metal in the oxide. The aluminium removes oxygen from the iron (iii) oxide: Iron(iii) oxide + aluminium → aluminium. Iron Iii Oxide Reacts With Aluminum.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID Iron Iii Oxide Reacts With Aluminum Aluminum replaces the metal in the oxide. This shows that aluminium is above iron in the reactivity series. This mixture of aluminum and iron oxide is called. Given below is the chemical. The aluminium removes oxygen from the iron (iii) oxide: Mohammad razak of the city college of new york's chemistry department shows us a. Iron(iii) oxide + aluminium →. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED The thermite reaction occurs when iron(III) oxide reacts with Iron Iii Oxide Reacts With Aluminum Given below is the chemical. This is because aluminum is more reactive than iron. Iron(iii) oxide + aluminium → aluminium oxide + iron. Reactions between metals and metal. In the reaction between aluminum and iron(iii) oxide, this forms iron and. The aluminium removes oxygen from the iron (iii) oxide: Aluminum replaces the metal in the oxide. This shows that aluminium. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED If of molten iron(III) oxide reacts with of aluminum, what is Iron Iii Oxide Reacts With Aluminum Reactions between metals and metal. Iron(iii) oxide + aluminium → aluminium oxide + iron. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Aluminum replaces the metal in the oxide. In the reaction between aluminum and iron(iii) oxide, this forms iron and. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3). Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED Al2O3 + 2Fe ——> Fe2O3 + 2Al How many moles of Iron (III) oxide Iron Iii Oxide Reacts With Aluminum A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Mohammad razak of the city college of new york's chemistry department shows us a. Reactions between metals and metal. The aluminium removes oxygen from the iron (iii) oxide: Given below is the chemical. Iron (iii) oxide. Iron Iii Oxide Reacts With Aluminum.
From www.solutionspile.com
[Solved] Aluminum metal reacts with Iron(III) oxide to pr Iron Iii Oxide Reacts With Aluminum Reactions between metals and metal. Given below is the chemical. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Aluminum replaces the metal in the oxide. This is because aluminum is more reactive than iron. The aluminium removes oxygen from the iron (iii) oxide: Mohammad. Iron Iii Oxide Reacts With Aluminum.
From www.youtube.com
Al+Fe2O3=Al2O3+Fe Balanced EquationAluminum+Iron(III) oxide+Iron Iron Iii Oxide Reacts With Aluminum Iron(iii) oxide + aluminium → aluminium oxide + iron. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Mohammad razak of the city college of new york's chemistry department shows us a. A thermite reaction or process is an exothermic redox reaction between. Iron Iii Oxide Reacts With Aluminum.
From wizedu.com
If 87.5g of molten iron (III) oxide reacts with 25.7g of aluminum Iron Iii Oxide Reacts With Aluminum The aluminium removes oxygen from the iron (iii) oxide: Mohammad razak of the city college of new york's chemistry department shows us a. This is because aluminum is more reactive than iron. Aluminum replaces the metal in the oxide. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting. Iron Iii Oxide Reacts With Aluminum.
From questions.kunduz.com
When iron(III) oxide reacts with aluminum... Physical Chemistry Iron Iii Oxide Reacts With Aluminum This shows that aluminium is above iron in the reactivity series. Iron(iii) oxide + aluminium → aluminium oxide + iron. This is because aluminum is more reactive than iron. Given below is the chemical. Reactions between metals and metal. The aluminium removes oxygen from the iron (iii) oxide: This mixture of aluminum and iron oxide is called. Aluminum replaces the. Iron Iii Oxide Reacts With Aluminum.
From www.youtube.com
Thermit reaction iron (III) oxide reacts with aluminium and gives Iron Iii Oxide Reacts With Aluminum This shows that aluminium is above iron in the reactivity series. Mohammad razak of the city college of new york's chemistry department shows us a. This mixture of aluminum and iron oxide is called. The aluminium removes oxygen from the iron (iii) oxide: In the reaction between aluminum and iron(iii) oxide, this forms iron and. Iron (iii) oxide (f e2o3). Iron Iii Oxide Reacts With Aluminum.
From questions.kunduz.com
References Use the References to access i... Physical Chemistry Iron Iii Oxide Reacts With Aluminum Aluminum replaces the metal in the oxide. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). This shows that aluminium is above iron in the reactivity series.. Iron Iii Oxide Reacts With Aluminum.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Iii Oxide Reacts With Aluminum Reactions between metals and metal. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This mixture of aluminum and iron oxide is called. Iron(iii) oxide + aluminium. Iron Iii Oxide Reacts With Aluminum.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Iron Iii Oxide Reacts With Aluminum Mohammad razak of the city college of new york's chemistry department shows us a. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). Iron(iii) oxide + aluminium. Iron Iii Oxide Reacts With Aluminum.
From www.bartleby.com
Answered The thermite reaction occurs when… bartleby Iron Iii Oxide Reacts With Aluminum In the reaction between aluminum and iron(iii) oxide, this forms iron and. This is because aluminum is more reactive than iron. The aluminium removes oxygen from the iron (iii) oxide: Mohammad razak of the city college of new york's chemistry department shows us a. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well. Iron Iii Oxide Reacts With Aluminum.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download Iron Iii Oxide Reacts With Aluminum This mixture of aluminum and iron oxide is called. This shows that aluminium is above iron in the reactivity series. Iron(iii) oxide + aluminium → aluminium oxide + iron. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). Given below is the chemical. Aluminum replaces the metal in the oxide. Iron. Iron Iii Oxide Reacts With Aluminum.
From www.bartleby.com
Answered The reaction between aluminium and… bartleby Iron Iii Oxide Reacts With Aluminum Reactions between metals and metal. This is because aluminum is more reactive than iron. A thermite reaction or process is an exothermic redox reaction between iron (iii) oxide (fe 2 o 3) and aluminum (al) in powder form. This mixture of aluminum and iron oxide is called. Given below is the chemical. The aluminium removes oxygen from the iron (iii). Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide Reacts With Aluminum Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). Mohammad razak of the city college of new york's chemistry department shows us a. Iron(iii) oxide + aluminium → aluminium oxide + iron. Aluminum replaces the metal in the oxide. In the reaction between aluminum and iron(iii) oxide, this forms iron and.. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED The chemical equation below represents the reaction between Iron Iii Oxide Reacts With Aluminum Mohammad razak of the city college of new york's chemistry department shows us a. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. This is because aluminum is more reactive than iron. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form aluminium oxide (al2o3) and molten iron (fe). Reactions between metals and metal. Aluminum. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron Iii Oxide Reacts With Aluminum This is because aluminum is more reactive than iron. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Given below is the chemical. A thermite reaction or process is an. Iron Iii Oxide Reacts With Aluminum.
From www.chegg.com
Solved At high temperatures iron(III) oxide reacts with Iron Iii Oxide Reacts With Aluminum Mohammad razak of the city college of new york's chemistry department shows us a. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). This mixture of aluminum and iron oxide is called. Iron (iii) oxide (f e2o3) reacts with aluminium (al) to form. Iron Iii Oxide Reacts With Aluminum.
From brainly.com
The Thermite reaction reacts iron (III) oxide, Fe2O3 with aluminium Iron Iii Oxide Reacts With Aluminum Given below is the chemical. Iron(iii) oxide + aluminium → aluminium oxide + iron. This shows that aluminium is above iron in the reactivity series. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Iron (iii) oxide (f e2o3) reacts with aluminium (al). Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED Write the balanced chemical equations for the following Iron Iii Oxide Reacts With Aluminum Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). In the reaction between aluminum and iron(iii) oxide, this forms iron and. Given below is the chemical. Aluminum replaces the metal in the oxide. The aluminium removes oxygen from the iron (iii) oxide: Iron(iii). Iron Iii Oxide Reacts With Aluminum.
From kunduz.com
[ANSWERED] A scientist reacts some aluminum metal with some iron III Iron Iii Oxide Reacts With Aluminum Given below is the chemical. Aluminum replaces the metal in the oxide. The aluminium removes oxygen from the iron (iii) oxide: In the reaction between aluminum and iron(iii) oxide, this forms iron and. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Mohammad razak of the city college of new york's chemistry department shows us a.. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED Question 2 10 pts In a redox reaction, solid iron (III) oxide Iron Iii Oxide Reacts With Aluminum Given below is the chemical. This is because aluminum is more reactive than iron. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Aluminum replaces the metal in the oxide. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c).. Iron Iii Oxide Reacts With Aluminum.
From www.numerade.com
SOLVED Determine the heat evolved (in kJ) when 15.0 g of Aluminum Iron Iii Oxide Reacts With Aluminum Mohammad razak of the city college of new york's chemistry department shows us a. This mixture of aluminum and iron oxide is called. Iron (iii) oxide reacts with aluminium and gives molten iron and aluminium oxide. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron. Iron Iii Oxide Reacts With Aluminum.