Cfr Labeling Medical Devices . various sections of the qs regulation have an impact on labeling: § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. Name and place of business of manufacturer, packer or distributor. navigate by entering citations or phrases (eg: § 801.150 medical devices; (a) the label of a device in. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far).
from knconsultingandservices.com
navigate by entering citations or phrases (eg: § 801.1 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.150 medical devices; Name and place of business of manufacturer, packer or distributor. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). Section 21 cfr 820.80 (b) requires the inspection and. (a) the label of a device in. various sections of the qs regulation have an impact on labeling:
What is Labelling? Medical Device Consulting Company
Cfr Labeling Medical Devices § 801.150 medical devices; navigate by entering citations or phrases (eg: Section 21 cfr 820.80 (b) requires the inspection and. § 801.1 medical devices; (a) the label of a device in. various sections of the qs regulation have an impact on labeling: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.150 medical devices;
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Cfr Labeling Medical Devices (a) the label of a device in. navigate by entering citations or phrases (eg: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.150 medical devices; § 801.1 medical devices;. Cfr Labeling Medical Devices.
From www.flexo-graphics.com
Medical Device Labeling Medical Equipment Labels Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). navigate by entering citations or phrases (eg: (a) the label of a device in. § 801.150 medical devices; § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. various sections of the qs regulation have an impact on labeling: . Cfr Labeling Medical Devices.
From www.artfulcompliance.com
00093 21 CFR Part 801 Labeling (of Medical Devices) Cfr Labeling Medical Devices (a) the label of a device in. Section 21 cfr 820.80 (b) requires the inspection and. § 801.1 medical devices; various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: § 801.150 medical devices; Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining. Cfr Labeling Medical Devices.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Cfr Labeling Medical Devices navigate by entering citations or phrases (eg: § 801.150 medical devices; (a) the label of a device in. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. various sections of the. Cfr Labeling Medical Devices.
From www.greenlight.guru
21 CFR 801 Labeling Requirements Overview [Guide] Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). Section 21 cfr 820.80 (b) requires the inspection and. § 801.1 medical devices; (a) the label of a device in. § 801.150 medical devices; various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: Name. Cfr Labeling Medical Devices.
From www.slideshare.net
Quality System Requirements 21 CFR Part 820 and Labelling Requirement… Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). various sections of the qs regulation have an impact on labeling: § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. navigate by entering citations or phrases (eg: (a) the label of a device in. Name and place of business of. Cfr Labeling Medical Devices.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. labeling regulations pertaining to medical devices are found in the following parts. Cfr Labeling Medical Devices.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). (a) the label of a device in. navigate by entering citations or phrases (eg: Section 21 cfr 820.80 (b) requires the inspection and. § 801.1 medical devices; § 801.150 medical devices; labeling regulations pertaining to medical devices are found in the following parts. Cfr Labeling Medical Devices.
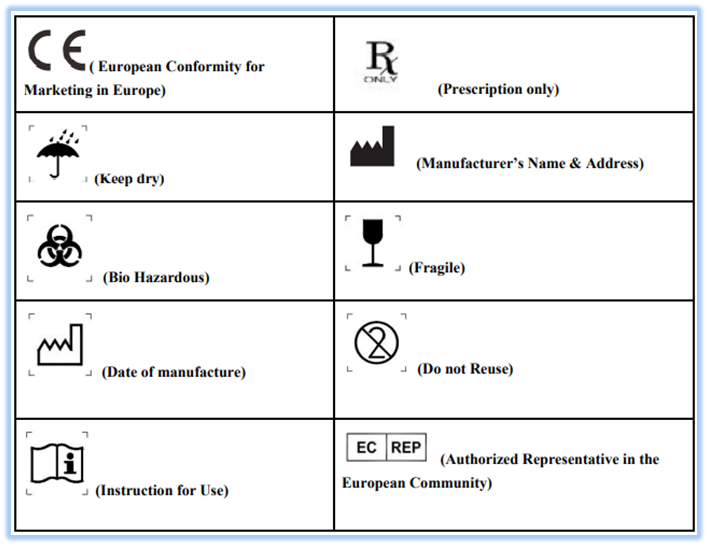
From mungfali.com
Medical Device Labeling Symbols Cfr Labeling Medical Devices § 801.1 medical devices; navigate by entering citations or phrases (eg: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Section 21 cfr 820.80 (b) requires the inspection and. § 801.150 medical devices; various sections of the qs regulation have an impact on. Cfr Labeling Medical Devices.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Cfr Labeling Medical Devices Name and place of business of manufacturer, packer or distributor. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. . Cfr Labeling Medical Devices.
From www.greenlight.guru
Ultimate Guide to 21 CFR Part 820 — FDA's Quality System Regulation Cfr Labeling Medical Devices Name and place of business of manufacturer, packer or distributor. various sections of the qs regulation have an impact on labeling: § 801.150 medical devices; navigate by entering citations or phrases (eg: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). (a) the label of a device in. § 801.1 medical devices;. Cfr Labeling Medical Devices.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Cfr Labeling Medical Devices § 801.1 medical devices; (a) the label of a device in. navigate by entering citations or phrases (eg: § 801.150 medical devices; various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b) requires the inspection and. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). . Cfr Labeling Medical Devices.
From giotbxaec.blob.core.windows.net
Software As A Medical Device Labeling Requirements at Steven Osborne blog Cfr Labeling Medical Devices § 801.150 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.1 medical devices; (a) the label of a device in. various sections of the qs regulation have an impact on labeling: 1 cfr 1.1 49 cfr 172.101 organization and purpose. Cfr Labeling Medical Devices.
From mungfali.com
Medical Device Labeling Symbols Cfr Labeling Medical Devices § 801.150 medical devices; § 801.1 medical devices; navigate by entering citations or phrases (eg: Section 21 cfr 820.80 (b) requires the inspection and. various sections of the qs regulation have an impact on labeling: Name and place of business of manufacturer, packer or distributor. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation. Cfr Labeling Medical Devices.
From www.presentationeze.com
FDA Medical Device Labeling.PresentationEZE Cfr Labeling Medical Devices navigate by entering citations or phrases (eg: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). various sections of the qs regulation have an impact on labeling: Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining to medical devices are found in the following parts of title 21 of. Cfr Labeling Medical Devices.
From www.artfulcompliance.com
21 CFR Part 860 Medical Device Classification Cfr Labeling Medical Devices various sections of the qs regulation have an impact on labeling: § 801.1 medical devices; navigate by entering citations or phrases (eg: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Section 21 cfr 820.80 (b) requires the inspection and. § 801.150 medical. Cfr Labeling Medical Devices.
From www.slideserve.com
PPT Overview of FDA Device Regulations PowerPoint Presentation, free Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). navigate by entering citations or phrases (eg: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. various sections of the qs regulation have an impact on labeling: § 801.1 medical devices;. Cfr Labeling Medical Devices.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Cfr Labeling Medical Devices navigate by entering citations or phrases (eg: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.150 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). various sections. Cfr Labeling Medical Devices.
From info.docxellent.com
FDA 21 CFR Part 820 Compliance for Medical Device Companies Cfr Labeling Medical Devices 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). navigate by entering citations or phrases (eg: § 801.1 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of manufacturer, packer or distributor. §. Cfr Labeling Medical Devices.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Cfr Labeling Medical Devices Section 21 cfr 820.80 (b) requires the inspection and. § 801.1 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. navigate by entering citations or phrases (eg: Name and place of business of manufacturer, packer or distributor. § 801.150 medical devices; (a). Cfr Labeling Medical Devices.
From www.youtube.com
21 CFR Part 820 Quality System Regulation 21 CFR 820.30 Medical Cfr Labeling Medical Devices § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: labeling regulations pertaining to medical devices are found in the following parts. Cfr Labeling Medical Devices.
From www.youtube.com
21 CFR Part 11 for Medical Device Manufacturers YouTube Cfr Labeling Medical Devices Name and place of business of manufacturer, packer or distributor. various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: § 801.1 medical devices; Section 21 cfr 820.80 (b) requires the inspection and. (a) the label of a device in. labeling regulations pertaining to medical devices are found. Cfr Labeling Medical Devices.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Cfr Labeling Medical Devices navigate by entering citations or phrases (eg: § 801.1 medical devices; various sections of the qs regulation have an impact on labeling: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). (a) the label of a device in. Name and place of business of manufacturer, packer or distributor. § 801.150 medical devices;. Cfr Labeling Medical Devices.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Cfr Labeling Medical Devices § 801.1 medical devices; navigate by entering citations or phrases (eg: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of manufacturer, packer or distributor. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). Section 21. Cfr Labeling Medical Devices.
From operonstrategist.com
US FDA 21 CFR 820.30 (Design Controls For Medical Devices) Operon Cfr Labeling Medical Devices labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. navigate by entering citations or phrases (eg: various sections of the qs regulation have an impact on labeling: § 801.1 medical devices; 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far).. Cfr Labeling Medical Devices.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Cfr Labeling Medical Devices Section 21 cfr 820.80 (b) requires the inspection and. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). navigate by entering citations or phrases (eg: § 801.1 medical devices; § 801.150 medical devices; various sections of the qs regulation have an impact on labeling: Name and place of business of manufacturer, packer. Cfr Labeling Medical Devices.
From peakvascularaccess.com
What is the meaning of symbols on medical devices labels? Peak Mobile Cfr Labeling Medical Devices various sections of the qs regulation have an impact on labeling: (a) the label of a device in. Section 21 cfr 820.80 (b) requires the inspection and. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1. Cfr Labeling Medical Devices.
From mungfali.com
Medical Device Labeling Symbols Cfr Labeling Medical Devices § 801.150 medical devices; Name and place of business of manufacturer, packer or distributor. (a) the label of a device in. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. various sections of the qs regulation have an impact on labeling: § 801.1 medical. Cfr Labeling Medical Devices.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Cfr Labeling Medical Devices labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: Name and place of business of manufacturer, packer or distributor. 1 cfr 1.1 49 cfr 172.101 organization and. Cfr Labeling Medical Devices.
From www.eleapsoftware.com
How an LMS Can Empower 21 CFR Part 11 Labeling Compliance Cfr Labeling Medical Devices Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. various sections of the qs regulation have an impact on labeling: § 801.150 medical devices; (a) the label of a device in. 1 cfr 1.1 49. Cfr Labeling Medical Devices.
From studylib.net
FDA Regulation 21CFR801 Medical Device Labeling Cfr Labeling Medical Devices various sections of the qs regulation have an impact on labeling: labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). Name and place of business of manufacturer, packer or distributor. (a) the label. Cfr Labeling Medical Devices.
From exytbaeqr.blob.core.windows.net
Medical Device Labeling Cfr at Joyce Richardson blog Cfr Labeling Medical Devices Name and place of business of manufacturer, packer or distributor. (a) the label of a device in. various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). § 801.1 medical devices; § 801.150 medical devices;. Cfr Labeling Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Cfr Labeling Medical Devices Section 21 cfr 820.80 (b) requires the inspection and. (a) the label of a device in. 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). § 801.1 medical devices; Name and place of business of manufacturer, packer or distributor. various sections of the qs regulation have an impact on labeling: labeling regulations pertaining. Cfr Labeling Medical Devices.
From qmsdoc.com
21 CFR Part 801 Labeling QMS Templates Cfr Labeling Medical Devices Name and place of business of manufacturer, packer or distributor. § 801.150 medical devices; (a) the label of a device in. navigate by entering citations or phrases (eg: 1 cfr 1.1 49 cfr 172.101 organization and purpose 1/1.1 regulation y far). various sections of the qs regulation have an impact on labeling: Section 21 cfr 820.80 (b). Cfr Labeling Medical Devices.
From www.youtube.com
FDA Requirements for Device Labeling YouTube Cfr Labeling Medical Devices labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. various sections of the qs regulation have an impact on labeling: navigate by entering citations or phrases (eg: Name and place of business of manufacturer, packer or distributor. (a) the label of a device in. Section. Cfr Labeling Medical Devices.