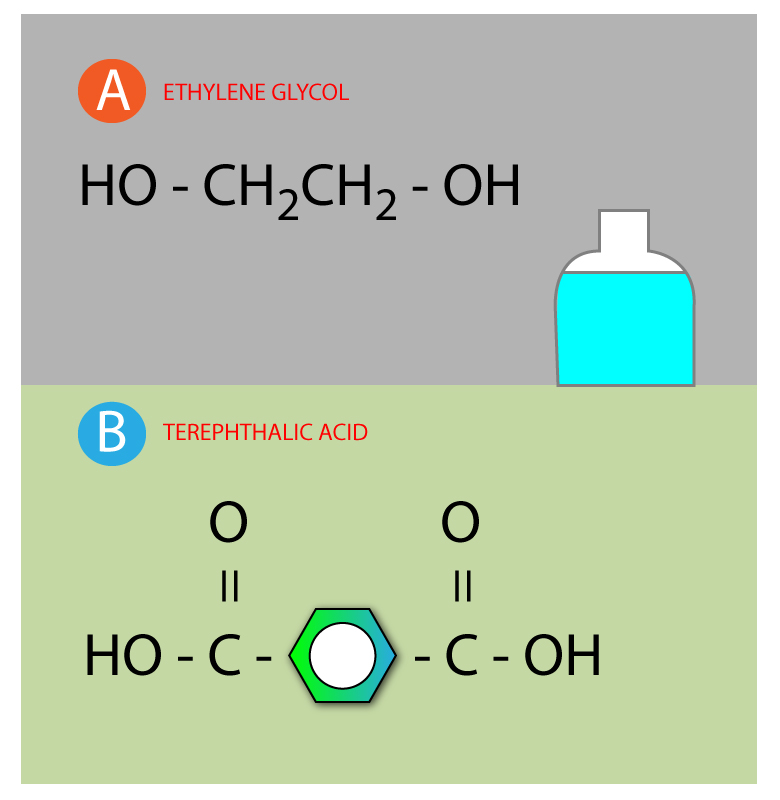
Examples Of The Condensation Polymer . Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. A simple polyester can be made from a monomer which has two hydroxyl.
from surfguppy.com
condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. A simple polyester can be made from a monomer which has two hydroxyl. polyesters are an example of a condensation polymer. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o.
Condensation Polymerization Surfguppy Chemistry made easy for
Examples Of The Condensation Polymer examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. A simple polyester can be made from a monomer which has two hydroxyl. polyesters are an example of a condensation polymer. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. Ethanol + ethanoic acid → ethyl ethanoate + water.
From www.slideserve.com
PPT Polymers PowerPoint Presentation, free download ID1460389 Examples Of The Condensation Polymer Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. polyesters are an example of a condensation polymer. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of. Examples Of The Condensation Polymer.
From marybrookz.blogspot.com
What Is Condensation Polymerization Explain With Example Mary Brook's Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ethanol. Examples Of The Condensation Polymer.
From www.youtube.com
Condensation polymers how is polyester made? YouTube Examples Of The Condensation Polymer polyesters are an example of a condensation polymer. A simple polyester can be made from a monomer which has two hydroxyl. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Condensation polymers PowerPoint Presentation, free download ID Examples Of The Condensation Polymer examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. Ch. Examples Of The Condensation Polymer.
From surfguppy.com
Condensation Polymerization Surfguppy Chemistry made easy for Examples Of The Condensation Polymer A simple polyester can be made from a monomer which has two hydroxyl. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. examples. Examples Of The Condensation Polymer.
From evulpo.com
Condensation polymers Chemistry Explanation & Exercises evulpo Examples Of The Condensation Polymer polyesters are an example of a condensation polymer. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains. Examples Of The Condensation Polymer.
From www.tes.com
Condensation Polymers Edexcel 91 Separate (Triple) Science by Examples Of The Condensation Polymer A simple polyester can be made from a monomer which has two hydroxyl. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that. Examples Of The Condensation Polymer.
From surfguppy.com
Condensation Polymerization Surfguppy Chemistry made easy for Examples Of The Condensation Polymer Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. polyesters are an example of a condensation polymer. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains. Examples Of The Condensation Polymer.
From www.youtube.com
Condensation Polymers (Part 1) YouTube Examples Of The Condensation Polymer Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid → ethyl ethanoate + water. A simple polyester can be made from a monomer which has two. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Chapter 15 Polymers Characteristics, Applications, and Examples Of The Condensation Polymer Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. polyesters are an example of a condensation polymer. A simple polyester can be made from a monomer which has two hydroxyl. . Examples Of The Condensation Polymer.
From www.slideserve.com
PPT ADDITION POLYMERS PowerPoint Presentation, free download ID1251418 Examples Of The Condensation Polymer examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. A simple polyester can be made from a monomer which has two hydroxyl. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid → ethyl ethanoate + water. polyesters are an example. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Condensation polymers PowerPoint Presentation, free download ID Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of naturally occurring condensation polymers are cellulose,. Examples Of The Condensation Polymer.
From www.chemistrystudent.com
Condensation Polymerisation (ALevel) ChemistryStudent Examples Of The Condensation Polymer examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid. Examples Of The Condensation Polymer.
From revisechemistry.uk
Synthetic and Naturally Occuring Polymers AQA C7 revisechemistry.uk Examples Of The Condensation Polymer Ethanol + ethanoic acid → ethyl ethanoate + water. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ch 3 ch 2 oh + ch 3 cooh. Examples Of The Condensation Polymer.
From www.tes.com
Condensation Polymers GCSE AQA Teaching Resources Examples Of The Condensation Polymer Ethanol + ethanoic acid → ethyl ethanoate + water. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. polyesters are an example of a condensation polymer. examples of naturally occurring condensation polymers are. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID3218044 Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. A simple polyester can be made from a monomer which has two hydroxyl. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Polymerization Reactions PowerPoint Presentation, free download Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. A simple polyester can be made from a monomer which has two hydroxyl. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2. Examples Of The Condensation Polymer.
From www.youtube.com
Polymers Condensation Polymerization YouTube Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch. Examples Of The Condensation Polymer.
From pediaa.com
Difference Between Addition Polymerisation and Condensation Examples Of The Condensation Polymer polyesters are an example of a condensation polymer. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. A. Examples Of The Condensation Polymer.
From marybrookz.blogspot.com
What Is Condensation Polymerization Explain With Example Mary Brook's Examples Of The Condensation Polymer examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. polyesters are an example of a condensation polymer. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β.. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Condensation polymers PowerPoint Presentation, free download ID Examples Of The Condensation Polymer Ethanol + ethanoic acid → ethyl ethanoate + water. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h. Examples Of The Condensation Polymer.
From shiken.ai
Condensation Polymers Examples Of The Condensation Polymer examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ethanol + ethanoic acid → ethyl ethanoate + water. A simple polyester can be made from a monomer which has two hydroxyl. examples. Examples Of The Condensation Polymer.
From www.youtube.com
Condensation Polymerisation GCSE Chemistry YouTube Examples Of The Condensation Polymer examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. polyesters are an example of a condensation polymer. Ethanol + ethanoic acid → ethyl ethanoate + water. A simple polyester can be made. Examples Of The Condensation Polymer.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. A simple polyester can be made from a monomer which has two hydroxyl. Ethanol + ethanoic acid → ethyl ethanoate +. Examples Of The Condensation Polymer.
From www.labster.com
5 Ways to Teach about Synthetic Polymers and Their Impact on Everyday Life Examples Of The Condensation Polymer Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. A simple polyester can be made from a monomer which has two hydroxyl. Ethanol + ethanoic acid → ethyl ethanoate +. Examples Of The Condensation Polymer.
From studyfurnishers.z4.web.core.windows.net
How To Draw Condensation Polymers Examples Of The Condensation Polymer polyesters are an example of a condensation polymer. A simple polyester can be made from a monomer which has two hydroxyl. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2. Examples Of The Condensation Polymer.
From almerja.net
Characteristics of Condensation Polymers Examples Of The Condensation Polymer polyesters are an example of a condensation polymer. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. Ethanol + ethanoic acid → ethyl ethanoate + water. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. Ch 3 ch 2 oh +. Examples Of The Condensation Polymer.
From www.britannica.com
Chemical reaction Polymerization, Monomers, Polymers Britannica Examples Of The Condensation Polymer polyesters are an example of a condensation polymer. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. A simple polyester can be made from a monomer which has two hydroxyl. Ch 3 ch 2 oh + ch. Examples Of The Condensation Polymer.
From www.onlinemathlearning.com
Condensation Polymers (examples, answers, activities, experiment, videos) Examples Of The Condensation Polymer examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. A simple polyester can be made from a monomer which has two hydroxyl. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3. Examples Of The Condensation Polymer.
From biology.reachingfordreams.com
Different types of synthetic polymers and their uses. Addition vs Examples Of The Condensation Polymer examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ch 3 ch 2 oh + ch 3 cooh →. Examples Of The Condensation Polymer.
From www.pdfprof.com
polymérisation par condensation Examples Of The Condensation Polymer examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. A simple polyester can be made from. Examples Of The Condensation Polymer.
From www.youtube.com
1. Condensation polymer defined (HSC chemistry) YouTube Examples Of The Condensation Polymer examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. Ch 3 ch 2 oh + ch 3 cooh →. Examples Of The Condensation Polymer.
From www.youtube.com
Ch 26 StepGrowth Polymers Condensation polymerization YouTube Examples Of The Condensation Polymer examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of natural. Examples Of The Condensation Polymer.
From byjus.com
How can deffrentiate addition polymer and condensation polymer? Examples Of The Condensation Polymer Ch 3 ch 2 oh + ch 3 cooh → ch 3 cooch 2 ch 3 + h 2 o. Ethanol + ethanoic acid → ethyl ethanoate + water. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. condensation polymers are formed through a chemical reaction that involves the elimination of. Examples Of The Condensation Polymer.
From www.science-revision.co.uk
Condensation polymers Examples Of The Condensation Polymer condensation polymers are formed through a chemical reaction that involves the elimination of a small molecule, such as. examples of natural condensation polymers include cellulose, starch, and polypeptide chains of proteins. examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly (β. polyesters are an example of a condensation polymer. Ch. Examples Of The Condensation Polymer.