Hard Acid Definition . what is a hard acid? hard acids are not very polarizable and have high charge densities. hsab is an acronym for hard and soft (lewis) acids and bases. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. Hard acids react faster with. hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. Thus metal ions with high positive charges and smaller ionic. Other things being approximately equal: Hsab is widely used in chemistry for explaining the. Hard acids are lewis acids that are only weakly polarizable. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower.
from mungfali.com
Hard acids are lewis acids that are only weakly polarizable. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. hsab is an acronym for hard and soft (lewis) acids and bases. hard acids are not very polarizable and have high charge densities. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. what is a hard acid? Hsab is widely used in chemistry for explaining the. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. Hard acids react faster with.
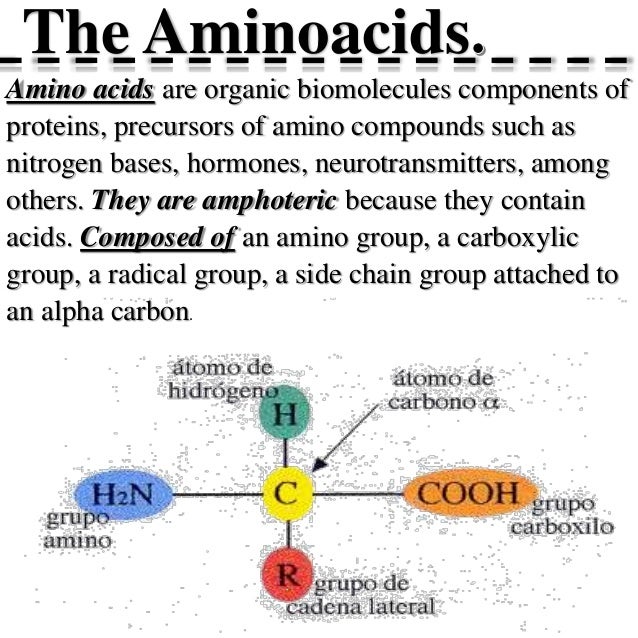
Functions Of Amino Acids
Hard Acid Definition Hard acids react faster with. what is a hard acid? metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Other things being approximately equal: hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. Hard acids react faster with. hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hsab is an acronym for hard and soft (lewis) acids and bases. Hsab is widely used in chemistry for explaining the. hard acids are not very polarizable and have high charge densities. Thus metal ions with high positive charges and smaller ionic. Hard acids are lewis acids that are only weakly polarizable.
From www.youtube.com
Hard Soft Acid Base Theory Professor Adam Teaches YouTube Hard Acid Definition hard acids are not very polarizable and have high charge densities. hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. what is a hard acid? Hard acids react faster with. Hsab is widely used in chemistry for explaining the. hard acids tend. Hard Acid Definition.
From atelier-yuwa.ciao.jp
What Is An Acid In Chemistry? Definition And Examples atelieryuwa Hard Acid Definition hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. Thus metal ions with high positive charges and smaller ionic. hsab is an acronym for hard and soft (lewis) acids and bases. Hard acids are lewis acids that are only weakly polarizable. hard acids are not very. Hard Acid Definition.
From fyoqutlma.blob.core.windows.net
What Does Iron (Iii) Oxide Mean at Anthony Silvernail blog Hard Acid Definition Hsab is widely used in chemistry for explaining the. what is a hard acid? a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. hard acids consist of small highly charged cations and molecules in which a high positive charge can be. Hard Acid Definition.
From sciencenotes.org
What Is a Nucleic Acid? Definition and Examples Hard Acid Definition hsab is an acronym for hard and soft (lewis) acids and bases. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Thus metal ions with high positive charges and smaller ionic. Hsab is widely used in chemistry for explaining the. Hard acids. Hard Acid Definition.
From studylib.net
Hard and Soft Acids and Bases Hard Acid Definition a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Other things being approximately equal: Hard acids are lewis acids that are only weakly polarizable. hard acids are not very polarizable and have high charge densities. hsab is an acronym for hard. Hard Acid Definition.
From www.slideserve.com
PPT HardSoft AcidBase Theory PowerPoint Presentation, free download Hard Acid Definition hsab is an acronym for hard and soft (lewis) acids and bases. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for. Hard Acid Definition.
From circuitenginerivage88.z22.web.core.windows.net
Water Ph Diagram Hard Acid Definition hsab is an acronym for hard and soft (lewis) acids and bases. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density.. Hard Acid Definition.
From www.chemistrysteps.com
Lewis Acids and Bases Chemistry Steps Hard Acid Definition hsab is an acronym for hard and soft (lewis) acids and bases. what is a hard acid? a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Hsab is widely used in chemistry for explaining the. metal ions with the highest. Hard Acid Definition.
From giodxtofs.blob.core.windows.net
To Make Proteins And Amino Acids at Warren Sutton blog Hard Acid Definition a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hsab is an acronym for hard and soft (lewis) acids and bases. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are.. Hard Acid Definition.
From exoazcenr.blob.core.windows.net
Nucleic Acid Definition In Plants at Mike Blackman blog Hard Acid Definition metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Thus metal ions with high positive charges and smaller ionic.. Hard Acid Definition.
From www.youtube.com
CHEM3006 28 Hard soft acid base theory examples YouTube Hard Acid Definition Thus metal ions with high positive charges and smaller ionic. Hsab is widely used in chemistry for explaining the. Hard acids react faster with. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hsab is an acronym for hard and soft (lewis) acids and bases.. Hard Acid Definition.
From www.sliderbase.com
HardSoft AcidBase Theory Presentation Chemistry Hard Acid Definition a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. Thus metal ions with high positive charges and smaller ionic. Hsab is widely used in chemistry for explaining the. Other things being approximately equal: hard acids consist of small highly charged cations and molecules in which. Hard Acid Definition.
From www.ndjtuition.com
Acid Bases and Salts Handwritten Notes for 10th Science Hard Acid Definition hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. hard acids are not very polarizable and have high. Hard Acid Definition.
From dokumen.tips
(PPT) HardSoft AcidBase Theory. Definitions Arrhenius acids form Hard Acid Definition hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. Hard acids react faster with. Other things being approximately equal: Hsab is widely used in. Hard Acid Definition.
From www.animalia-life.club
Nucleic Acid Hard Acid Definition what is a hard acid? Hard acids react faster with. Hard acids are lewis acids that are only weakly polarizable. hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. a hard or soft acid is a hard or soft lewis acid, and a. Hard Acid Definition.
From pediaa.com
Difference Between Acid and Base Hard Acid Definition Hard acids are lewis acids that are only weakly polarizable. Hsab is widely used in chemistry for explaining the. Hard acids react faster with. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. what is a hard acid? Thus metal ions with. Hard Acid Definition.
From www.studocu.com
Hard Acids Understanding the Concept in Chemistry A hard Hard Acid Definition a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. hard acids are not very polarizable and have high charge densities. hsab is an acronym for hard and soft (lewis) acids and bases. what is a hard acid? hard acids. Hard Acid Definition.
From ar.inspiredpencil.com
Strong Organic Acids List Hard Acid Definition hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. hard acids are not very polarizable and have high charge densities. what is a hard acid? hsab is an acronym for hard and soft (lewis) acids and bases. metal ions with the highest affinities for. Hard Acid Definition.
From cbseadda.blogspot.ae
CBSE Chemistry 7th Acids bases and Salts [Solved] CBSE ADDA Hard Acid Definition hard acids are not very polarizable and have high charge densities. Other things being approximately equal: hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft. Hard Acid Definition.
From fyocllxdv.blob.core.windows.net
Define Fatty Acid In Chemistry at Kent Bass blog Hard Acid Definition Hsab is widely used in chemistry for explaining the. Other things being approximately equal: Hard acids are lewis acids that are only weakly polarizable. Hard acids react faster with. Thus metal ions with high positive charges and smaller ionic. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower.. Hard Acid Definition.
From loeoxecye.blob.core.windows.net
Chemical Acids List at Maria Carr blog Hard Acid Definition hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. a hard or soft acid is a hard or soft lewis acid, and a. Hard Acid Definition.
From www.teachoo.com
Equal lengths of magnesium ribbons are taken in test tubes A and B Hard Acid Definition Thus metal ions with high positive charges and smaller ionic. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base.. Hard Acid Definition.
From loeoxecye.blob.core.windows.net
Chemical Acids List at Maria Carr blog Hard Acid Definition hsab is an acronym for hard and soft (lewis) acids and bases. Other things being approximately equal: a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hard acids are not very polarizable and have high charge densities. Hsab is widely used in chemistry for. Hard Acid Definition.
From www.slideserve.com
PPT HardSoft AcidBase Theory PowerPoint Presentation, free download Hard Acid Definition hsab is an acronym for hard and soft (lewis) acids and bases. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. Hsab is widely used in chemistry for explaining the. Thus metal ions with high positive charges and smaller ionic. Hard acids are lewis. Hard Acid Definition.
From animalia-life.club
Common Acid Hcl Hard Acid Definition Thus metal ions with high positive charges and smaller ionic. hsab is an acronym for hard and soft (lewis) acids and bases. Hard acids react faster with. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hard acids consist of small highly charged cations. Hard Acid Definition.
From www.slideserve.com
PPT chemistry B.Sc III PowerPoint Presentation, free Hard Acid Definition Hard acids are lewis acids that are only weakly polarizable. hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hsab is. Hard Acid Definition.
From www.deviantart.com
Hard Acid by TechBehr on DeviantArt Hard Acid Definition a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. hard acids are not very polarizable and have high charge densities. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft. Hard Acid Definition.
From www.difference.wiki
Hard Acid vs. Soft Acid What’s the Difference? Hard Acid Definition a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. Hard acids are lewis acids that are only weakly polarizable. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. . Hard Acid Definition.
From mungfali.com
Functions Of Amino Acids Hard Acid Definition Thus metal ions with high positive charges and smaller ionic. metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. a hard acid is a type of lewis acid that is characterized by having a small size and a high charge density. hard acids. Hard Acid Definition.
From hxeultexy.blob.core.windows.net
Nucleic Acid Element Formula at Marianne Grosz blog Hard Acid Definition metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for soft bases are. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. Hard acids react faster with. a hard acid is a type of lewis acid. Hard Acid Definition.
From exomkcrdf.blob.core.windows.net
Nucleic Acids Function Biomolecules at Almeda Rivera blog Hard Acid Definition hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom. Hard acids are lewis acids that are only weakly polarizable. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. what is a hard. Hard Acid Definition.
From study.com
Lewis Acid & Base Definition & Examples Lesson Hard Acid Definition Thus metal ions with high positive charges and smaller ionic. Hard acids react faster with. Hsab is widely used in chemistry for explaining the. what is a hard acid? a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Hard acids are lewis. Hard Acid Definition.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Hard Acid Definition Hsab is widely used in chemistry for explaining the. Other things being approximately equal: a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and. Hard Acid Definition.
From dk.pinterest.com
Radiographer, Radiographer Poster, Radiographer Definition Hard Acid Definition hard acids tend to have smaller atomic radii and higher electronegativity, while soft acids have larger atomic radii and lower. hard acids are not very polarizable and have high charge densities. what is a hard acid? metal ions with the highest affinities for hard bases are hard acids, whereas metal ions with the highest affinity for. Hard Acid Definition.
From www.worksheetsplanet.com
What is an Acid Definition of Acid Hard Acid Definition Hard acids are lewis acids that are only weakly polarizable. Hsab is widely used in chemistry for explaining the. a hard or soft acid is a hard or soft lewis acid, and a hard or soft base is a hard or soft lewis base. Thus metal ions with high positive charges and smaller ionic. a hard acid is. Hard Acid Definition.