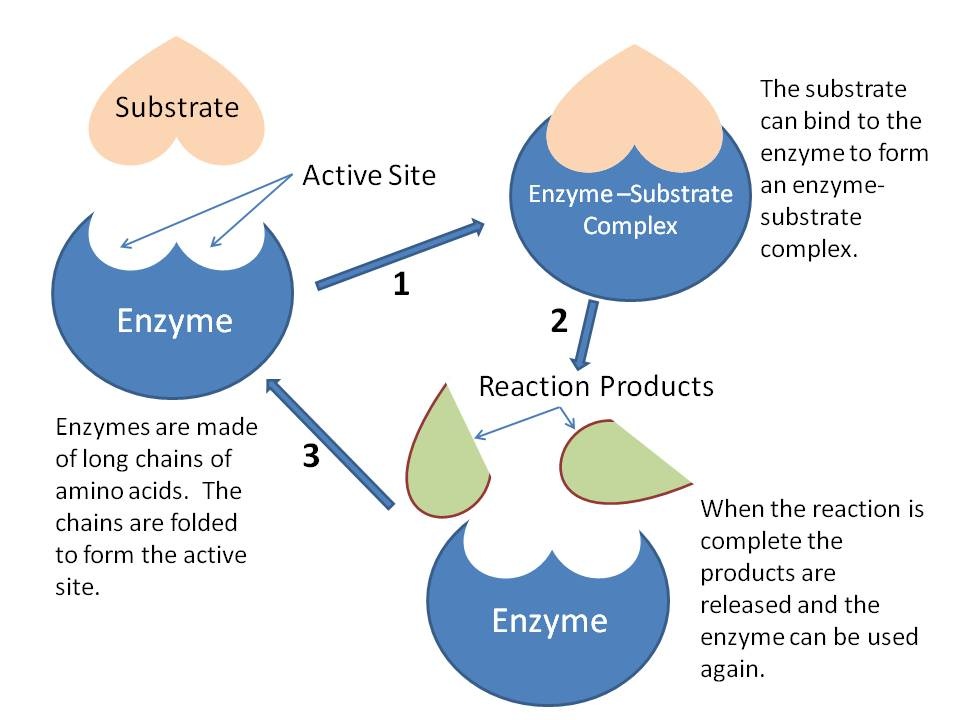
How Do Enzymes Lower Activation Energy And Increase Reaction Rate . the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. enzymes lower the activation energy necessary to transform a reactant into a product. How do enzymes speed up biochemical reactions so dramatically? enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction that is not catalyzed by an enzyme. Like all catalysts, enzymes work by lowering the activation. One of the ways the activation.
from dxotdtire.blob.core.windows.net
the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). How do enzymes speed up biochemical reactions so dramatically? enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. One of the ways the activation. On the left is a reaction that is not catalyzed by an enzyme. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. Like all catalysts, enzymes work by lowering the activation.
Enzymes Function In Chemical Reactions To at Susanne Anderson blog
How Do Enzymes Lower Activation Energy And Increase Reaction Rate Like all catalysts, enzymes work by lowering the activation. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. How do enzymes speed up biochemical reactions so dramatically? On the left is a reaction that is not catalyzed by an enzyme. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). Like all catalysts, enzymes work by lowering the activation. enzymes can lower the activation energy of a chemical reaction in three ways. One of the ways the activation. enzymes lower the activation energy necessary to transform a reactant into a product. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition.
From www.slideserve.com
PPT ENZYMES PowerPoint Presentation, free download ID6886656 How Do Enzymes Lower Activation Energy And Increase Reaction Rate On the left is a reaction that is not catalyzed by an enzyme. enzymes lower the activation energy necessary to transform a reactant into a product. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). How do enzymes speed up biochemical reactions so dramatically? the addition of. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From exodjtdpy.blob.core.windows.net
Enzymes Raise The Activation Energy Of A Reaction at Sean Ferguson blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). How do enzymes speed up biochemical reactions so dramatically? One of the ways the activation. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. Like all catalysts, enzymes work by lowering. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT Endergonic and Exergonic Reactions PowerPoint Presentation ID How Do Enzymes Lower Activation Energy And Increase Reaction Rate Like all catalysts, enzymes work by lowering the activation. How do enzymes speed up biochemical reactions so dramatically? On the left is a reaction that is not catalyzed by an enzyme. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition.. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From exozqvxvu.blob.core.windows.net
How Does An Enzyme Affect Delta G at Judith Heldt blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). enzymes lower the activation energy necessary to transform a reactant. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.pinterest.ca
Enzymes Draw It to Know It Biochemistry, Teaching biology, Biology How Do Enzymes Lower Activation Energy And Increase Reaction Rate the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. enzymes lower the activation energy necessary to transform a reactant into a product. the addition of a catalyst,. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.genome.gov
Enzyme How Do Enzymes Lower Activation Energy And Increase Reaction Rate catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. How do enzymes speed up biochemical reactions so dramatically? enzymes. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From dxotdtire.blob.core.windows.net
Enzymes Function In Chemical Reactions To at Susanne Anderson blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. How do enzymes speed up biochemical reactions so dramatically? Like all catalysts, enzymes work by lowering the activation. On the left is a reaction that is not catalyzed by an enzyme. . How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT AS Biology PowerPoint Presentation, free download ID2454000 How Do Enzymes Lower Activation Energy And Increase Reaction Rate we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. Like all catalysts, enzymes work by lowering the activation. enzymes can lower the activation energy of a chemical reaction in three ways. catalysts lower the activation energy, which is the amount of energy required for reactants. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From exozqvxvu.blob.core.windows.net
How Does An Enzyme Affect Delta G at Judith Heldt blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate Like all catalysts, enzymes work by lowering the activation. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition.. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 How Do Enzymes Lower Activation Energy And Increase Reaction Rate How do enzymes speed up biochemical reactions so dramatically? On the left is a reaction that is not catalyzed by an enzyme. Like all catalysts, enzymes work by lowering the activation. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). enzymes lower the activation energy necessary to transform. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From present5.com
Enzyme Structure classification and mechanism of action How Do Enzymes Lower Activation Energy And Increase Reaction Rate How do enzymes speed up biochemical reactions so dramatically? enzymes can lower the activation energy of a chemical reaction in three ways. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. catalysts lower the activation energy, which is the amount of energy required for reactants. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.chegg.com
Solved How Enzymes Function The graph presents three How Do Enzymes Lower Activation Energy And Increase Reaction Rate enzymes can lower the activation energy of a chemical reaction in three ways. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 How Do Enzymes Lower Activation Energy And Increase Reaction Rate the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). enzymes can lower the activation energy of a chemical reaction in three ways. we will see that enzymes employ various. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From exyerwjum.blob.core.windows.net
How Do Enzymes Reduce Activation Energy at Pamela Edwards blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate How do enzymes speed up biochemical reactions so dramatically? One of the ways the activation. enzymes lower the activation energy necessary to transform a reactant into a product. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. we will see that enzymes employ various chemical strategies to increase the. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From animalia-life.club
Enzyme Activation Energy Graph How Do Enzymes Lower Activation Energy And Increase Reaction Rate we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. Like all catalysts, enzymes work by lowering the activation. On the left is a reaction that is not catalyzed by an enzyme. the addition of a catalyst, an agent that speeds up the rate of a reaction. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 How Do Enzymes Lower Activation Energy And Increase Reaction Rate How do enzymes speed up biochemical reactions so dramatically? One of the ways the activation. enzymes lower the activation energy necessary to transform a reactant into a product. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. Like all catalysts, enzymes work by lowering the activation. we will see. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free How Do Enzymes Lower Activation Energy And Increase Reaction Rate One of the ways the activation. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). Like all catalysts, enzymes work by lowering the activation. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction,. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii How Do Enzymes Lower Activation Energy And Increase Reaction Rate On the left is a reaction that is not catalyzed by an enzyme. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. How do enzymes speed up biochemical reactions. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From dxotdtire.blob.core.windows.net
Enzymes Function In Chemical Reactions To at Susanne Anderson blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. enzymes can lower the activation energy of a chemical reaction in three ways. Like all catalysts, enzymes work by lowering the activation. catalysts lower the activation energy, which is the amount of energy required for reactants. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From philschatz.com
Chemical Reactions · Anatomy and Physiology How Do Enzymes Lower Activation Energy And Increase Reaction Rate enzymes lower the activation energy necessary to transform a reactant into a product. How do enzymes speed up biochemical reactions so dramatically? we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. One of the ways the activation. On the left is a reaction that is not. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 How Do Enzymes Lower Activation Energy And Increase Reaction Rate enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction that is not catalyzed by an enzyme. enzymes lower the activation energy necessary to transform a reactant into a product. How do enzymes speed up biochemical reactions so dramatically? we will see that enzymes employ various chemical strategies. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From ar.inspiredpencil.com
Enzymes Activation Energy How Do Enzymes Lower Activation Energy And Increase Reaction Rate How do enzymes speed up biochemical reactions so dramatically? catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). One of the ways the activation. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. the addition. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner How Do Enzymes Lower Activation Energy And Increase Reaction Rate the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. One of the ways the activation. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. Like all catalysts, enzymes work by lowering. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.chegg.com
Solved Question 25Which of these do enzymes do to increase How Do Enzymes Lower Activation Energy And Increase Reaction Rate enzymes lower the activation energy necessary to transform a reactant into a product. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). One of the ways the activation. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www2.nau.edu
Enzymes and Reaction Rates How Do Enzymes Lower Activation Energy And Increase Reaction Rate the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. How do enzymes speed up biochemical reactions so dramatically?. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From www.slideserve.com
PPT Metabolism PowerPoint Presentation, free download ID1153506 How Do Enzymes Lower Activation Energy And Increase Reaction Rate How do enzymes speed up biochemical reactions so dramatically? the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. the exact mechanism whereby the enzyme acts to increase the rate of the reaction differs from one. catalysts lower the. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From animalia-life.club
Enzyme Activation Energy Graph How Do Enzymes Lower Activation Energy And Increase Reaction Rate the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. On the left is a reaction that is not catalyzed by an enzyme. enzymes lower the activation energy necessary to transform a reactant into a product. the exact mechanism. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From ar.inspiredpencil.com
Enzymes Activation Energy How Do Enzymes Lower Activation Energy And Increase Reaction Rate the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction, provides an alternative transition. catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). How do enzymes speed up biochemical reactions so dramatically? enzymes. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From loemeimcs.blob.core.windows.net
Effects Enzymes Have On Substrates at Jean Coleman blog How Do Enzymes Lower Activation Energy And Increase Reaction Rate catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). On the left is a reaction that is not catalyzed by an enzyme. One of the ways the activation. enzymes lower the activation energy necessary to transform a reactant into a product. enzymes can lower the activation energy. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From anaskassidy.blogspot.com
36+ How Enzymes Work Diagram AnasKassidy How Do Enzymes Lower Activation Energy And Increase Reaction Rate catalysts lower the activation energy, which is the amount of energy required for reactants to form products (figure 1). One of the ways the activation. Like all catalysts, enzymes work by lowering the activation. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. enzymes can. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.
From studylib.net
Enzymes Lower Activation Energy How Do Enzymes Lower Activation Energy And Increase Reaction Rate On the left is a reaction that is not catalyzed by an enzyme. we will see that enzymes employ various chemical strategies to increase the rates of reactions, in addition to physical ones like. the addition of a catalyst, an agent that speeds up the rate of a reaction but is not consumed or altered by the reaction,. How Do Enzymes Lower Activation Energy And Increase Reaction Rate.