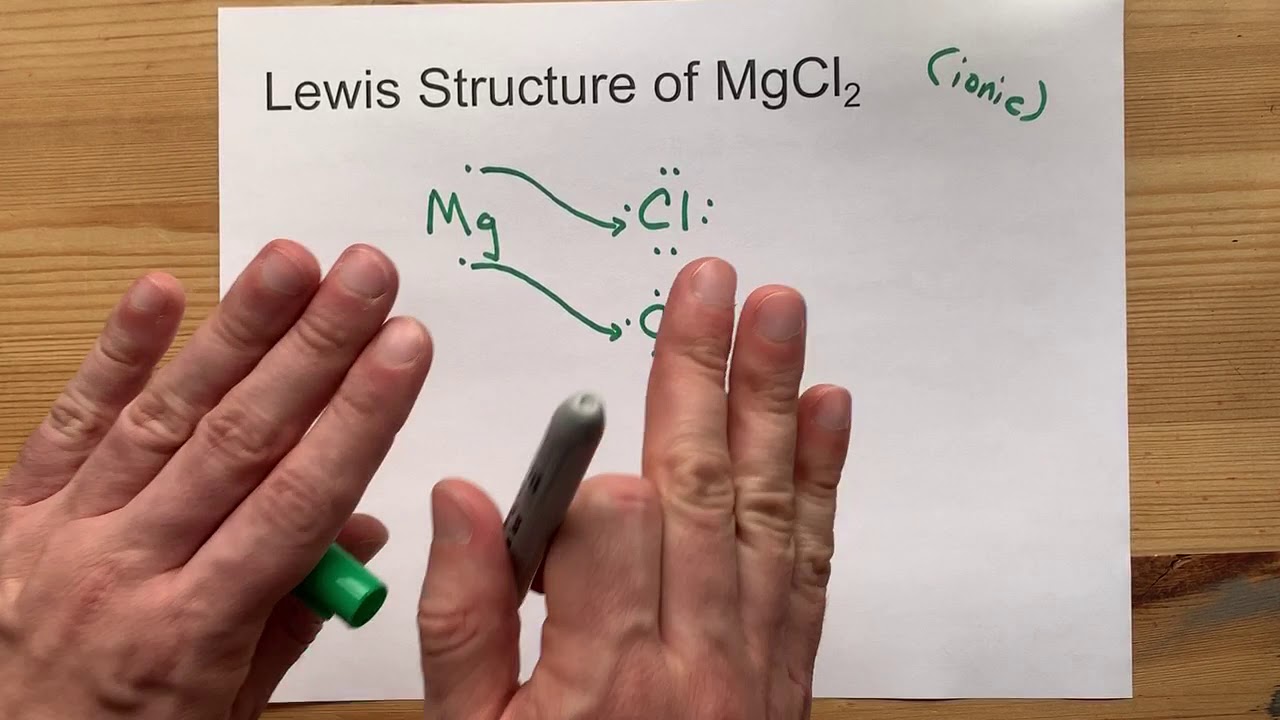
Magnesium Chloride Free Electrons . Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A chlorine atom will gain 1 electron to form. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Chlorine is in group 7 of the periodic table. Magnesium ions gain electrons to form magnesium atoms. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed from its elements. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. The chlorine atoms combine to form molecules of chlorine gas. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. These 2 electrons are transferred to and.
from www.youtube.com
Chlorine is in group 7 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 + ion. These 2 electrons are transferred to and. Magnesium ions gain electrons to form magnesium atoms. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed from its elements. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. The chlorine atoms combine to form molecules of chlorine gas.
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube
Magnesium Chloride Free Electrons These 2 electrons are transferred to and. A chlorine atom will gain 1 electron to form. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Magnesium ions gain electrons to form magnesium atoms. These 2 electrons are transferred to and. Chlorine is in group 7 of the periodic table. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed from its elements. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. The chlorine atoms combine to form molecules of chlorine gas.
From www.science-revision.co.uk
Introduction to ionic bonding Magnesium Chloride Free Electrons Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Magnesium ions gain electrons to form magnesium atoms. Chlorine is in group 7 of the periodic table. The chlorine atoms combine to form molecules of chlorine gas. Mg + cl = mgcl2 is a synthesis reaction where one mole. Magnesium Chloride Free Electrons.
From brainly.in
How is magnesium chloride formed by transfer of electrons ?why does the Magnesium Chloride Free Electrons These 2 electrons are transferred to and. Chlorine is in group 7 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed from its elements. Under these conditions, the ions close ion electrically. Magnesium Chloride Free Electrons.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Magnesium Chloride Free Electrons The chlorine atoms combine to form molecules of chlorine gas. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium chloride is formed from its elements. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium.. Magnesium Chloride Free Electrons.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride Free Electrons Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. These 2 electrons are transferred to and. The chlorine atoms combine to form molecules of chlorine gas. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to. Magnesium Chloride Free Electrons.
From circuitlibbottega.z21.web.core.windows.net
Correct Electron Dot Diagram For Magnesium Magnesium Chloride Free Electrons Chlorine is in group 7 of the periodic table. Magnesium ions gain electrons to form magnesium atoms. A magnesium atom will lose 2 electrons to form a stable 2 + ion. A chlorine atom will gain 1 electron to form. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. The. Magnesium Chloride Free Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Chloride Free Electrons Chlorine is in group 7 of the periodic table. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. These 2 electrons are transferred to and. Draw a diagram, with. Magnesium Chloride Free Electrons.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Magnesium Chloride Free Electrons A magnesium atom will lose 2 electrons to form a stable 2 + ion. The chlorine atoms combine to form molecules of chlorine gas. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A chlorine atom will gain 1 electron to form. Chloride ions lose electrons ( oxidation ) to. Magnesium Chloride Free Electrons.
From loegrhpel.blob.core.windows.net
Magnesium Electron Configuration Full at Ronny Robb blog Magnesium Chloride Free Electrons A magnesium atom will lose 2 electrons to form a stable 2 + ion. A chlorine atom will gain 1 electron to form. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium. Magnesium Chloride Free Electrons.
From brainly.in
How is magnesium chloride formed by the transfer of electrons why does Magnesium Chloride Free Electrons The chlorine atoms combine to form molecules of chlorine gas. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A chlorine atom will gain 1 electron to form. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Under these conditions, the ions close ion electrically. Magnesium Chloride Free Electrons.
From www.toppr.com
Draw electron dot representation for the formation of magnesium chloride. Magnesium Chloride Free Electrons Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. Chlorine is in group 7 of the periodic table. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. Magnesium ions gain electrons. Magnesium Chloride Free Electrons.
From www.edplace.com
Explain Ionic bonding Worksheet EdPlace Magnesium Chloride Free Electrons Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Magnesium ions gain electrons to form magnesium atoms. Chlorine is in group 7 of the periodic table. A magnesium atom will lose 2 electrons to form a stable 2 +. Magnesium Chloride Free Electrons.
From favpng.com
Lewis Structure Magnesium Chloride Electron Diagram, PNG, 1024x1024px Magnesium Chloride Free Electrons A magnesium atom will lose 2 electrons to form a stable 2 + ion. The chlorine atoms combine to form molecules of chlorine gas. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Magnesium ions gain electrons to form magnesium atoms. These 2 electrons are transferred to and.. Magnesium Chloride Free Electrons.
From nursehub.com
Electron Shells NurseHub Magnesium Chloride Free Electrons A magnesium atom will lose 2 electrons to form a stable 2 + ion. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Chlorine is in group 7 of the periodic table. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two. Magnesium Chloride Free Electrons.
From www.dreamstime.com
3D Image of Magnesium Chloride Skeletal Formula Stock Illustration Magnesium Chloride Free Electrons Chlorine is in group 7 of the periodic table. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. The chlorine atoms combine to form molecules of chlorine gas. These 2 electrons are transferred to and. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. A chlorine. Magnesium Chloride Free Electrons.
From www.toppr.com
Magnesium combines with chlorine to form magnesium chloride. The Magnesium Chloride Free Electrons Chlorine is in group 7 of the periodic table. Magnesium ions gain electrons to form magnesium atoms. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. A magnesium atom will lose 2 electrons to form a stable 2 + ion. These 2 electrons are transferred to and. Magnesium atoms. Magnesium Chloride Free Electrons.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID651377 Magnesium Chloride Free Electrons Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine. Magnesium Chloride Free Electrons.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride Free Electrons A magnesium atom will lose 2 electrons to form a stable 2 + ion. The chlorine atoms combine to form molecules of chlorine gas. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium. Magnesium Chloride Free Electrons.
From slideplayer.com
30/11/2018 nrt. ppt download Magnesium Chloride Free Electrons Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A chlorine atom will gain 1 electron to form. Chlorine is in group. Magnesium Chloride Free Electrons.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Magnesium Chloride Free Electrons Magnesium ions gain electrons to form magnesium atoms. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Each magnesium ion is surrounded by two chloride. Magnesium Chloride Free Electrons.
From www.toppr.com
Give orbital diagram of the followingmagnesium chloride, Magnesium Chloride Free Electrons Magnesium ions gain electrons to form magnesium atoms. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses. Magnesium Chloride Free Electrons.
From slideplayer.com
Overview Bonding, Structure and the properties of matter ppt download Magnesium Chloride Free Electrons Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A chlorine atom will gain 1 electron to form. Chlorine is in group 7 of the periodic table. Chloride ions. Magnesium Chloride Free Electrons.
From brainly.in
I) show the formation of magnesium chloride and sodium chloride by Magnesium Chloride Free Electrons A chlorine atom will gain 1 electron to form. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Chlorine is in group 7 of the periodic table. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains. Magnesium Chloride Free Electrons.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Magnesium Chloride Free Electrons Magnesium ions gain electrons to form magnesium atoms. These 2 electrons are transferred to and. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Chlorine is in group 7 of the periodic table. Magnesium atoms lose two. Magnesium Chloride Free Electrons.
From slideplayer.com
Stable Electron Configurations ppt download Magnesium Chloride Free Electrons Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. These 2 electrons are transferred to and. A chlorine atom will gain 1 electron to form. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium. Magnesium Chloride Free Electrons.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Chloride Free Electrons The chlorine atoms combine to form molecules of chlorine gas. A chlorine atom will gain 1 electron to form. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Magnesium. Magnesium Chloride Free Electrons.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation ID598959 Magnesium Chloride Free Electrons Magnesium ions gain electrons to form magnesium atoms. Chlorine is in group 7 of the periodic table. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. A magnesium atom will lose 2 electrons to form a stable 2 + ion. The chlorine atoms combine to form molecules of chlorine gas.. Magnesium Chloride Free Electrons.
From brainly.in
how is magnesium chloride formed by the transfer of electron why does Magnesium Chloride Free Electrons The chlorine atoms combine to form molecules of chlorine gas. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Draw a diagram, with outer electrons only, to show how the electrons are transferred when magnesium. Magnesium Chloride Free Electrons.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Magnesium Chloride Free Electrons The chlorine atoms combine to form molecules of chlorine gas. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of magnesium. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Under these conditions, the ions close ion electrically charged particle, formed. Magnesium Chloride Free Electrons.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Chloride Free Electrons Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. A chlorine atom will gain 1 electron to form.. Magnesium Chloride Free Electrons.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium Chloride Free Electrons A chlorine atom will gain 1 electron to form. Magnesium atoms lose two electrons from its outer shell, thus form positvely charged ions with a charge of 2+. The chlorine atoms combine to form molecules of chlorine gas. Chlorine is in group 7 of the periodic table. Magnesium ions gain electrons to form magnesium atoms. Draw a diagram, with outer. Magnesium Chloride Free Electrons.
From joittsmyb.blob.core.windows.net
Magnesium Lewis Electron Dot Structure at Diane Gilbert blog Magnesium Chloride Free Electrons A magnesium atom will lose 2 electrons to form a stable 2 + ion. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. Draw a diagram, with outer. Magnesium Chloride Free Electrons.
From www.slideserve.com
PPT Visualizing a Chemical Reaction PowerPoint Presentation, free Magnesium Chloride Free Electrons Chlorine is in group 7 of the periodic table. These 2 electrons are transferred to and. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. A magnesium atom will lose 2 electrons to form a stable 2 +. Magnesium Chloride Free Electrons.
From brainly.in
Write the formation of magnesium chloride (MgCl,) with the help of Magnesium Chloride Free Electrons Magnesium ions gain electrons to form magnesium atoms. A magnesium atom will lose 2 electrons to form a stable 2 + ion. Under these conditions, the ions close ion electrically charged particle, formed when an atom or molecule gains or loses electrons. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine. Magnesium Chloride Free Electrons.
From schematicsetwall.z14.web.core.windows.net
Correct Electron Dot Diagram For Magnesium Magnesium Chloride Free Electrons These 2 electrons are transferred to and. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. A chlorine atom will gain 1 electron to form. Chlorine is in group 7 of the periodic table. Magnesium ions gain electrons to form magnesium atoms. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium. Magnesium Chloride Free Electrons.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride Free Electrons These 2 electrons are transferred to and. Chloride ions lose electrons ( oxidation ) to form chlorine atoms. Each magnesium ion is surrounded by two chloride ions due to the 1:2 ratio of magnesium to chlorine in the compound. A chlorine atom will gain 1 electron to form. Draw a diagram, with outer electrons only, to show how the electrons. Magnesium Chloride Free Electrons.