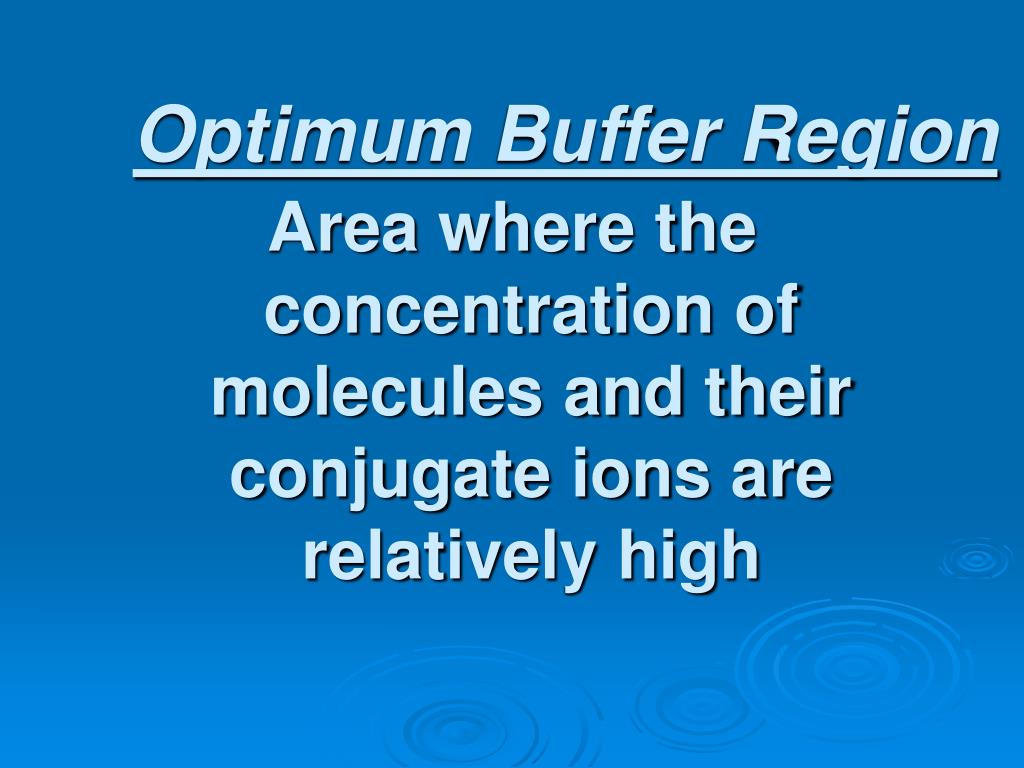
What Is Buffer Region . Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is formed containing excess ammonia and ammonium chloride. It is able to neutralize small. At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
from www.slideserve.com
A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. At the beginning of the titration the ph is much lower. A buffer solution is formed containing excess ammonia and ammonium chloride.
PPT COMMON ION EFFECT PowerPoint Presentation, free download ID6209182
What Is Buffer Region Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. At the beginning of the titration the ph is much lower. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
From saylordotorg.github.io
Buffers What Is Buffer Region Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is a solution that maintains the stability of a. What Is Buffer Region.
From www.troutheadwaters.com
What is a "Riparian Buffer Zone" and Why is it Important? What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. At the. What Is Buffer Region.
From www.chegg.com
Solved Consider the following questions about buffer What Is Buffer Region At the beginning of the titration the ph is much lower. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is Buffer Region.
From www.researchgate.net
Buffer zones from the major roads Download Scientific Diagram What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. It is able to neutralize small.. What Is Buffer Region.
From www.slideshare.net
Buffer system What Is Buffer Region A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of. What Is Buffer Region.
From www.slideserve.com
PPT Buffer Zones Distances, Credits & Posting (2011) PowerPoint What Is Buffer Region Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small. What Is Buffer Region.
From www.researchgate.net
Structure of central, inner buffer, and outer buffer regions in the What Is Buffer Region A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. At the beginning of the titration the ph is much lower. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that. What Is Buffer Region.
From mavink.com
Buffer Region Titration Curve What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer solution is formed containing excess ammonia and ammonium chloride. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph. What Is Buffer Region.
From www.youtube.com
Titration Curves for High School Chemistry YouTube What Is Buffer Region A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. At the beginning of the titration the ph is much lower. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer regions are the areas on a titration. What Is Buffer Region.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is Buffer Region A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. At the beginning of the titration the ph is much lower. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change. What Is Buffer Region.
From www.slideserve.com
PPT Buffer Zones Distances, Credits & Posting (2011) PowerPoint What Is Buffer Region At the beginning of the titration the ph is much lower. It is able to neutralize small. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change. What Is Buffer Region.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is formed containing excess ammonia and ammonium chloride. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of. What Is Buffer Region.
From www.slideserve.com
PPT Marine Ecosystems Vocabulary PowerPoint Presentation, free What Is Buffer Region A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. At the beginning of the titration the ph is much lower. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions. What Is Buffer Region.
From landscapedna.org
LandscapeDNA What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. At the beginning of the titration the ph is much lower. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that can resist ph change upon the addition of. What Is Buffer Region.
From www.russellconveyor.com
Buffer Zone Basics the Simplified Truth Russell Conveyor What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer solution is formed containing. What Is Buffer Region.
From www.vedantu.com
Core zone, buffer zone and manipulation zone are found in(a) Tiger What Is Buffer Region A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is formed containing excess ammonia and ammonium chloride. It is able to neutralize small. At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change. What Is Buffer Region.
From www.slideserve.com
PPT COMMON ION EFFECT PowerPoint Presentation, free download ID6209182 What Is Buffer Region It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. At the beginning of the titration the ph is much lower. Buffer regions. What Is Buffer Region.
From www.slideserve.com
PPT Chapter 5 Volumetric Analysis PowerPoint Presentation, free What Is Buffer Region Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. It is able to neutralize small. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is Buffer Region.
From www.youtube.com
The Buffer Region YouTube What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of. What Is Buffer Region.
From thinkwildlifefoundation.com
What are Buffer Zones and why are they important? What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer solution is formed containing excess ammonia and ammonium chloride. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that can resist ph change upon the addition of. What Is Buffer Region.
From mungfali.com
Buffer Region On Titration Curve What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. At the beginning of the titration the ph is much lower. It is able to neutralize small. A buffer is a solution that maintains the. What Is Buffer Region.
From www.youtube.com
NCEA L3 Chem Buffers, Buffer Region & Preparation of Buffer Solutions What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration. What Is Buffer Region.
From mavink.com
Buffer Region Titration Curve What Is Buffer Region A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding small. What Is Buffer Region.
From mavink.com
Buffer Region Titration Curve What Is Buffer Region A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. At the beginning of the titration the ph is much lower. A buffer is a solution that. What Is Buffer Region.
From ar.inspiredpencil.com
Titration Curve Buffer Region What Is Buffer Region A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. At the beginning of the titration the ph. What Is Buffer Region.
From quizdborienteers.z4.web.core.windows.net
What Is Buffer Region What Is Buffer Region Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. It is able to neutralize small. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is Buffer Region.
From ar.inspiredpencil.com
Titration Curve Buffer Region What Is Buffer Region A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding small. What Is Buffer Region.
From quizdboenometers.z13.web.core.windows.net
What Is Buffer Region What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. At the beginning of the titration the ph is much lower. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change. What Is Buffer Region.
From www.springfieldmo.gov
Regulations Springfield, MO Official site What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer regions. What Is Buffer Region.
From collegedunia.com
Buffer Region Working and Examples What Is Buffer Region A buffer solution is formed containing excess ammonia and ammonium chloride. At the beginning of the titration the ph is much lower. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of. What Is Buffer Region.
From www.reddit.com
If a buffer region is a region which resists change in pH, why isn’t What Is Buffer Region At the beginning of the titration the ph is much lower. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that can resist ph change upon the addition of. What Is Buffer Region.
From www.myxxgirl.com
Buffer Region Titration Curve My XXX Hot Girl What Is Buffer Region It is able to neutralize small. A buffer solution is formed containing excess ammonia and ammonium chloride. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong. What Is Buffer Region.
From www.slideserve.com
PPT Titration PowerPoint Presentation ID3996730 What Is Buffer Region At the beginning of the titration the ph is much lower. A buffer solution is formed containing excess ammonia and ammonium chloride. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. It is able to neutralize small. A buffer is a solution that maintains the. What Is Buffer Region.
From www.chegg.com
Solved Which point in the graph represents a buffer region? What Is Buffer Region A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is formed containing excess ammonia and ammonium chloride. Buffer regions. What Is Buffer Region.
From quizdborienteers.z4.web.core.windows.net
What Is Buffer Region What Is Buffer Region At the beginning of the titration the ph is much lower. It is able to neutralize small. A buffer solution is formed containing excess ammonia and ammonium chloride. Buffer regions are the areas on a titration curve where the ph changes very little despite the addition of a strong acid or base. A buffer is a solution that maintains the. What Is Buffer Region.