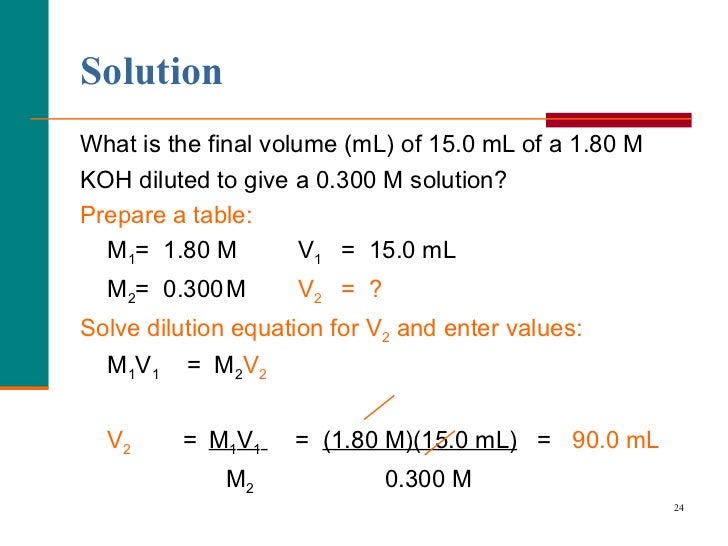
In A Dilution Process The Volume And The Molarity ___ . Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Learn how to dilute and concentrate solutions. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. According to the definition of molarity, the molar. Process of adding solvent to a solution in order to lower the concentration of solutes. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Describes the process by which solute components are dispersed in a.
from www.slideshare.net
Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Learn how to dilute and concentrate solutions. Process of adding solvent to a solution in order to lower the concentration of solutes. According to the definition of molarity, the molar. Dilution is the addition of. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Describes the process by which solute components are dispersed in a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
Molarity and dilution
In A Dilution Process The Volume And The Molarity ___ The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Describes the process by which solute components are dispersed in a. According to the definition of molarity, the molar. Learn how to dilute and concentrate solutions. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the addition of. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Process of adding solvent to a solution in order to lower the concentration of solutes. State whether the concentration of a solution is directly or indirectly proportional to its volume.
From www.slideshare.net
Molarity and dilution In A Dilution Process The Volume And The Molarity ___ Process of adding solvent to a solution in order to lower the concentration of solutes. Describes the process by which solute components are dispersed in a. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. The solute concentration of a solution may be decreased by. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Molarity and Dilution Solution Chemistry. ppt download In A Dilution Process The Volume And The Molarity ___ According to the definition of molarity, the molar. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. State whether the concentration of a solution is directly or indirectly. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Molarity and Dilutions ppt download In A Dilution Process The Volume And The Molarity ___ Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Describes the process by which solute components are dispersed in a. A simple mathematical relationship can be used. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Part I SOLUTION STOICHIOMETRY ppt download In A Dilution Process The Volume And The Molarity ___ According to the definition of molarity, the molar. Learn how to dilute and concentrate solutions. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of a solution is directly. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Molarity Chemistry Tutorial YouTube In A Dilution Process The Volume And The Molarity ___ State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. According to the definition of molarity, the molar. Process of adding solvent to a solution in order to lower the concentration. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Molarity and Dilution Calculations YouTube In A Dilution Process The Volume And The Molarity ___ Dilution is the addition of. According to the definition of molarity, the molar. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Learn how to dilute and concentrate solutions. Describes the process by which solute components are dispersed in a. Dilution is the process of “lowering the. In A Dilution Process The Volume And The Molarity ___.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts In A Dilution Process The Volume And The Molarity ___ Dilution is the addition of. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Learn how to dilute and concentrate solutions. According to the definition of molarity, the molar. Process of adding solvent to a solution in order to lower the concentration of solutes. State whether the. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Molarity and Dilutions ppt download In A Dilution Process The Volume And The Molarity ___ Dilution is the addition of. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Learn how to dilute and concentrate solutions. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. According to. In A Dilution Process The Volume And The Molarity ___.
From www.slideserve.com
PPT Molarity and Dilutions PowerPoint Presentation, free download In A Dilution Process The Volume And The Molarity ___ Describes the process by which solute components are dispersed in a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Process of adding solvent to a solution in order to lower the concentration of solutes. The solute concentration of a solution may be decreased by adding solvent,. In A Dilution Process The Volume And The Molarity ___.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet In A Dilution Process The Volume And The Molarity ___ Describes the process by which solute components are dispersed in a. State whether the concentration of a solution is directly or indirectly proportional to its volume. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. The solute concentration of a solution may be decreased by adding solvent,. In A Dilution Process The Volume And The Molarity ___.
From www.slideshare.net
Molarity and dilution In A Dilution Process The Volume And The Molarity ___ Learn how to dilute and concentrate solutions. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Process of adding solvent to a solution in order to lower the concentration of solutes.. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Molarity Dilution Practice Problem 3 YouTube In A Dilution Process The Volume And The Molarity ___ Dilution is the addition of. Process of adding solvent to a solution in order to lower the concentration of solutes. Learn how to dilute and concentrate solutions. According to the definition of molarity, the molar. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Describes the process by which solute components. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Chapter 8 Solutions 8.5 Molarity and Dilution. ppt download In A Dilution Process The Volume And The Molarity ___ Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. State whether the concentration of a solution is directly or indirectly proportional to its volume. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. A simple mathematical. In A Dilution Process The Volume And The Molarity ___.
From www.slideserve.com
PPT Molarity and Dilutions PowerPoint Presentation, free download In A Dilution Process The Volume And The Molarity ___ Describes the process by which solute components are dispersed in a. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. According to the definition of molarity, the molar. Process of adding solvent to a solution in order to lower the concentration of solutes. Learn how to dilute and concentrate solutions. Often,. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Molarity and Dilution YouTube In A Dilution Process The Volume And The Molarity ___ The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. According to the definition of molarity, the molar. State whether the concentration of a solution is directly or indirectly proportional to its volume. Process of adding solvent to a solution in order to lower the concentration of solutes. Dilution is the process. In A Dilution Process The Volume And The Molarity ___.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet In A Dilution Process The Volume And The Molarity ___ Describes the process by which solute components are dispersed in a. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the addition of. Learn how to dilute and concentrate solutions. The solute concentration of a solution may be decreased by adding solvent, a. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Molarity and Dilutions ppt download In A Dilution Process The Volume And The Molarity ___ Learn how to dilute and concentrate solutions. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Describes the process by which solute components are dispersed in a. Dilution. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Molarity and Dilutions ppt download In A Dilution Process The Volume And The Molarity ___ According to the definition of molarity, the molar. Describes the process by which solute components are dispersed in a. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Process of adding solvent to a solution in order to lower the concentration of solutes. A simple mathematical relationship can be used to. In A Dilution Process The Volume And The Molarity ___.
From www.slideshare.net
Molarity and dilution In A Dilution Process The Volume And The Molarity ___ The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples In A Dilution Process The Volume And The Molarity ___ Process of adding solvent to a solution in order to lower the concentration of solutes. State whether the concentration of a solution is directly or indirectly proportional to its volume. Describes the process by which solute components are dispersed in a. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
U10L4 Molarity, Dilution, PPM, and Molality Calculations YouTube In A Dilution Process The Volume And The Molarity ___ Dilution is the addition of. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Process of adding solvent to a solution in order to lower the concentration of solutes. According to the definition of molarity, the molar. State whether the concentration of a solution is directly or. In A Dilution Process The Volume And The Molarity ___.
From www.slideserve.com
PPT Molarity and Dilutions PowerPoint Presentation, free download In A Dilution Process The Volume And The Molarity ___ A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The solute concentration of a. In A Dilution Process The Volume And The Molarity ___.
From www.slideserve.com
PPT Molarity and Dilutions PowerPoint Presentation, free download In A Dilution Process The Volume And The Molarity ___ The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Dilution is the addition of. Dilution is the process of “lowering the concentration of a solute in a solution by. In A Dilution Process The Volume And The Molarity ___.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID5724692 In A Dilution Process The Volume And The Molarity ___ Process of adding solvent to a solution in order to lower the concentration of solutes. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. Learn how to dilute and concentrate solutions. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution.. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Molarity How to solve dilution problems Chemtutorfree YouTube In A Dilution Process The Volume And The Molarity ___ State whether the concentration of a solution is directly or indirectly proportional to its volume. Describes the process by which solute components are dispersed in a. Dilution is the addition of. Learn how to dilute and concentrate solutions. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Dilution is the process. In A Dilution Process The Volume And The Molarity ___.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality In A Dilution Process The Volume And The Molarity ___ According to the definition of molarity, the molar. Learn how to dilute and concentrate solutions. Dilution is the addition of. Describes the process by which solute components are dispersed in a. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. A simple mathematical relationship can be used to relate the volumes. In A Dilution Process The Volume And The Molarity ___.
From www.slideshare.net
Molarity and dilution In A Dilution Process The Volume And The Molarity ___ Describes the process by which solute components are dispersed in a. According to the definition of molarity, the molar. Learn how to dilute and concentrate solutions. Process of adding solvent to a solution in order to lower the concentration of solutes. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its. In A Dilution Process The Volume And The Molarity ___.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet In A Dilution Process The Volume And The Molarity ___ Dilution is the addition of. Describes the process by which solute components are dispersed in a. According to the definition of molarity, the molar. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
Solutions Dilution by Molarity YouTube In A Dilution Process The Volume And The Molarity ___ State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. Learn how to dilute and. In A Dilution Process The Volume And The Molarity ___.
From www.youtube.com
DILUTION AND MIXING OF SOLUTIONS, MOLARITY EQUATION /SOME BASIC In A Dilution Process The Volume And The Molarity ___ According to the definition of molarity, the molar. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Process of adding solvent to a solution in order to lower the concentration of solutes. Dilution. In A Dilution Process The Volume And The Molarity ___.
From www.slideshare.net
Molarity and dilution In A Dilution Process The Volume And The Molarity ___ Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Process of adding solvent to a solution in order to lower the concentration of solutes. According to the definition of molarity, the molar. Dilution is the process of “lowering the concentration of a solute in. In A Dilution Process The Volume And The Molarity ___.
From slideplayer.com
Review of Basic Concepts, Molarity, Solutions and Dilutions ppt download In A Dilution Process The Volume And The Molarity ___ Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. According to the definition of molarity, the molar. Process of adding solvent to a solution in order to lower the concentration of solutes. The solute concentration of a solution may be decreased by adding solvent, a. In A Dilution Process The Volume And The Molarity ___.
From www.slideshare.net
Molarity Molality Dilutions In A Dilution Process The Volume And The Molarity ___ Process of adding solvent to a solution in order to lower the concentration of solutes. Describes the process by which solute components are dispersed in a. According to the definition of molarity, the molar. A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. State whether the concentration. In A Dilution Process The Volume And The Molarity ___.
From www.slideserve.com
PPT Molarity & Dilution PowerPoint Presentation, free download ID In A Dilution Process The Volume And The Molarity ___ Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Describes the process by which solute components are dispersed in a.. In A Dilution Process The Volume And The Molarity ___.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis In A Dilution Process The Volume And The Molarity ___ The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Describes the process by which solute components are dispersed in a. State whether the concentration of a. In A Dilution Process The Volume And The Molarity ___.