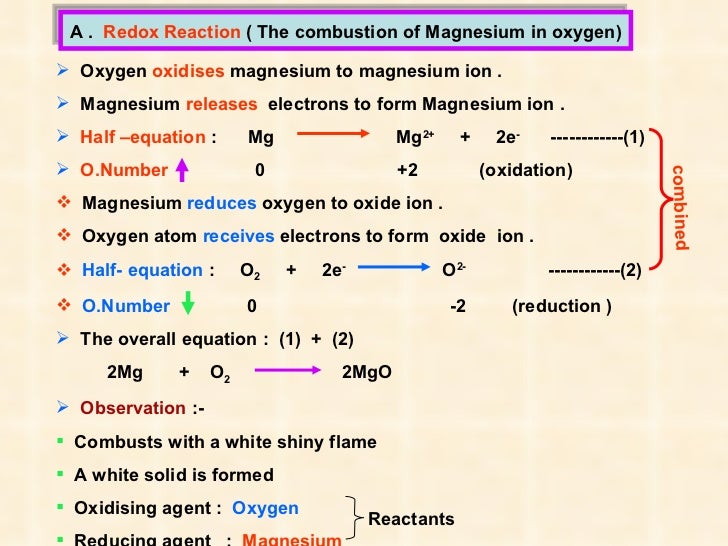
Magnesium Carbonate Oxidation Number . Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). A net ionic charge can be specified at the end of the. The oxidation state of magnesium has increased from 0 to +2; Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of hydrogen has. The element has been oxidized. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons.
from articleeducation.x.fc2.com
Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of magnesium has increased from 0 to +2; A net ionic charge can be specified at the end of the. The element has been oxidized. The oxidation state of hydrogen has.
Explain how magnesium and oxygen atoms react to form magnesium oxide
Magnesium Carbonate Oxidation Number Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. The oxidation state of magnesium has increased from 0 to +2; Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of hydrogen has. The element has been oxidized. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons.
From socratic.org
Which one of the elements with following outer electronic Magnesium Carbonate Oxidation Number Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). A net ionic charge can be specified at the end of the. The oxidation state of hydrogen has. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of magnesium has increased from 0 to. Magnesium Carbonate Oxidation Number.
From rodolfozebrivera.blogspot.com
Oxidation Number of Magnesium RodolfozebRivera Magnesium Carbonate Oxidation Number A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation state of hydrogen has. Knowing the oxidation number of each individual element in a molecule will be a. Magnesium Carbonate Oxidation Number.
From slideplayer.com
Chapter 20 Review “OxidationReduction Reactions” ppt download Magnesium Carbonate Oxidation Number The element has been oxidized. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). Enter the formula of a chemical compound to find the oxidation number of each element. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of hydrogen has. The oxidation. Magnesium Carbonate Oxidation Number.
From www.periodni.com
Oxidationszahlen Rechner Magnesium Carbonate Oxidation Number The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. A net ionic charge can be specified at the end of the. The element has been oxidized. Enter the formula of a chemical compound to find the oxidation number of each element.. Magnesium Carbonate Oxidation Number.
From www.youtube.com
Oxidation reaction of Magnesium Reaction of Magnesium with Oxygen Magnesium Carbonate Oxidation Number The oxidation state of hydrogen has. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. The oxidation state of magnesium has increased from 0 to +2; The oxidation number of each atom can be calculated by subtracting the sum of lone pairs. Magnesium Carbonate Oxidation Number.
From www.youtube.com
Magnesium oxidation number in Mg3N2 ( magnesium nitride ) YouTube Magnesium Carbonate Oxidation Number Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation state of magnesium has increased from 0 to +2; Enter the formula of a chemical compound to find the. Magnesium Carbonate Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for C in MgCO3 (Magnesium carbonate Magnesium Carbonate Oxidation Number The oxidation state of hydrogen has. A net ionic charge can be specified at the end of the. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Knowing the oxidation. Magnesium Carbonate Oxidation Number.
From bmp-brah.blogspot.com
Mgco3 Balanced Equation bmpbrah Magnesium Carbonate Oxidation Number Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. The oxidation state of hydrogen has. The oxidation state of magnesium has. Magnesium Carbonate Oxidation Number.
From www.dreamstime.com
Magnesium Carbonate Molecule Stock Vector Illustration of Magnesium Carbonate Oxidation Number A net ionic charge can be specified at the end of the. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence. Magnesium Carbonate Oxidation Number.
From www.youtube.com
20 g of magnesium carbonate sample on heating to give carbon Magnesium Carbonate Oxidation Number The oxidation state of hydrogen has. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of magnesium has increased from 0 to +2; Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation number of each atom can be calculated by subtracting. Magnesium Carbonate Oxidation Number.
From www.slideserve.com
PPT Reaction of Magnesium with Carbon Dioxide PowerPoint Presentation Magnesium Carbonate Oxidation Number Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation state of hydrogen has. The oxidation state of magnesium has increased from 0 to +2; The oxidation number of each atom can be calculated by subtracting. Magnesium Carbonate Oxidation Number.
From www.tessshebaylo.com
Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of hydrogen has. The element has been oxidized. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. A net ionic charge can be specified at the end of the. The. Magnesium Carbonate Oxidation Number.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of magnesium has increased from 0 to +2; The oxidation state of hydrogen has. A net ionic charge can be specified. Magnesium Carbonate Oxidation Number.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Magnesium Carbonate Oxidation Number Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The element has been oxidized. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. A net ionic charge can be specified at the end of the. The oxidation state of magnesium has increased from 0 to +2;. Magnesium Carbonate Oxidation Number.
From www.youtube.com
Formation of Magnesium Oxide (MgO) Chemical Bonding Atomic Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of magnesium has increased from 0 to +2; A net ionic charge can be specified at the end of the. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The. Magnesium Carbonate Oxidation Number.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Carbonate Oxidation Number The oxidation state of hydrogen has. The oxidation state of magnesium has increased from 0 to +2; Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. A net ionic charge can be specified at the end of the. Calculate the oxidation number of each element in mgco3*nh2o (magnesium. Magnesium Carbonate Oxidation Number.
From www.chegg.com
Solved of magnesium carbonate produces Magnesium Carbonate Oxidation Number The oxidation state of magnesium has increased from 0 to +2; The oxidation state of hydrogen has. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. The element has been oxidized. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate).. Magnesium Carbonate Oxidation Number.
From www.glentham.com
Magnesium carbonate, basic, chemical pure (CAS 39409820) Glentham Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. A net ionic charge can be specified at the end of the. The element has been oxidized. The oxidation state of hydrogen has. The. Magnesium Carbonate Oxidation Number.
From www.youtube.com
Lewis Structure of Magnesium Oxide (MgO) YouTube Magnesium Carbonate Oxidation Number The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. A net ionic charge can be specified at the end of the. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation state of hydrogen has. The element has. Magnesium Carbonate Oxidation Number.
From www.slideserve.com
PPT Elements and Periodic Table PowerPoint Presentation, free Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of magnesium has increased from 0 to +2; The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Calculate the oxidation number of each. Magnesium Carbonate Oxidation Number.
From www.slideshare.net
Metals, Non Metals And Oxidation Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. The oxidation state of magnesium has increased from 0 to +2; Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The element has been oxidized. The oxidation state of hydrogen has.. Magnesium Carbonate Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for C in CH3COOH (Acetic acid ) YouTube Magnesium Carbonate Oxidation Number A net ionic charge can be specified at the end of the. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of. Magnesium Carbonate Oxidation Number.
From articleeducation.x.fc2.com
Explain how magnesium and oxygen atoms react to form magnesium oxide Magnesium Carbonate Oxidation Number The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate).. Magnesium Carbonate Oxidation Number.
From www.slideserve.com
PPT Valence Electrons and Oxidation Numbers PowerPoint Presentation Magnesium Carbonate Oxidation Number The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation state of hydrogen has. A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each. Magnesium Carbonate Oxidation Number.
From byjus.com
20.0 g of a magnesium carbonate sample on heating to give Magnesium Carbonate Oxidation Number The oxidation state of magnesium has increased from 0 to +2; Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons.. Magnesium Carbonate Oxidation Number.
From www.researchgate.net
XRD patterns of various magnesium carbonate synthesized at different Magnesium Carbonate Oxidation Number The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Enter the formula of a chemical compound to find the oxidation number of each element. The element has been oxidized. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The. Magnesium Carbonate Oxidation Number.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Carbonate Oxidation Number The oxidation state of magnesium has increased from 0 to +2; Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation state of hydrogen has. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Knowing the oxidation number. Magnesium Carbonate Oxidation Number.
From honorassignme.co
📚 What is an example of oxidation number?, oxidation states examples Magnesium Carbonate Oxidation Number The element has been oxidized. The oxidation state of hydrogen has. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). Enter the formula of a chemical compound to find the oxidation number of each element. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. A net. Magnesium Carbonate Oxidation Number.
From www.fishersci.co.uk
Magnesium carbonate, for biochemistry, specified according to the Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation state of magnesium has increased from 0 to +2; Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The element has been oxidized. Knowing the oxidation number of each individual element in a molecule will be a key step in our. Magnesium Carbonate Oxidation Number.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID270027 Magnesium Carbonate Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. The element has been oxidized. A net ionic charge can be specified at the end of the. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation state of magnesium has increased from 0 to +2; The oxidation state of hydrogen has.. Magnesium Carbonate Oxidation Number.
From rodolfozebrivera.blogspot.com
Oxidation Number of Magnesium RodolfozebRivera Magnesium Carbonate Oxidation Number Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation state of magnesium has increased from 0 to +2; Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. The oxidation state of hydrogen has. The oxidation number of each atom can be calculated by subtracting. Magnesium Carbonate Oxidation Number.
From brainly.in
When 8.4 g of magnesium carbonate is heated, into Magnesium Carbonate Oxidation Number A net ionic charge can be specified at the end of the. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The oxidation state of magnesium has increased from 0. Magnesium Carbonate Oxidation Number.
From www.youtube.com
The molar solubility of magnesium carbonate is 1.87x10^4 mol/L Magnesium Carbonate Oxidation Number The element has been oxidized. Knowing the oxidation number of each individual element in a molecule will be a key step in our understanding of redox. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from. Magnesium Carbonate Oxidation Number.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Magnesium Carbonate Oxidation Number The oxidation state of magnesium has increased from 0 to +2; The oxidation state of hydrogen has. The element has been oxidized. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence. Magnesium Carbonate Oxidation Number.
From www.pinterest.com
Oxidation of Magnesium,Mg Magnesium, Oxidation, Oxidation state Magnesium Carbonate Oxidation Number The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Calculate the oxidation number of each element in mgco3*nh2o (magnesium carbonate). The oxidation state of hydrogen has. The element has been oxidized. Knowing the oxidation number of each individual element in a. Magnesium Carbonate Oxidation Number.