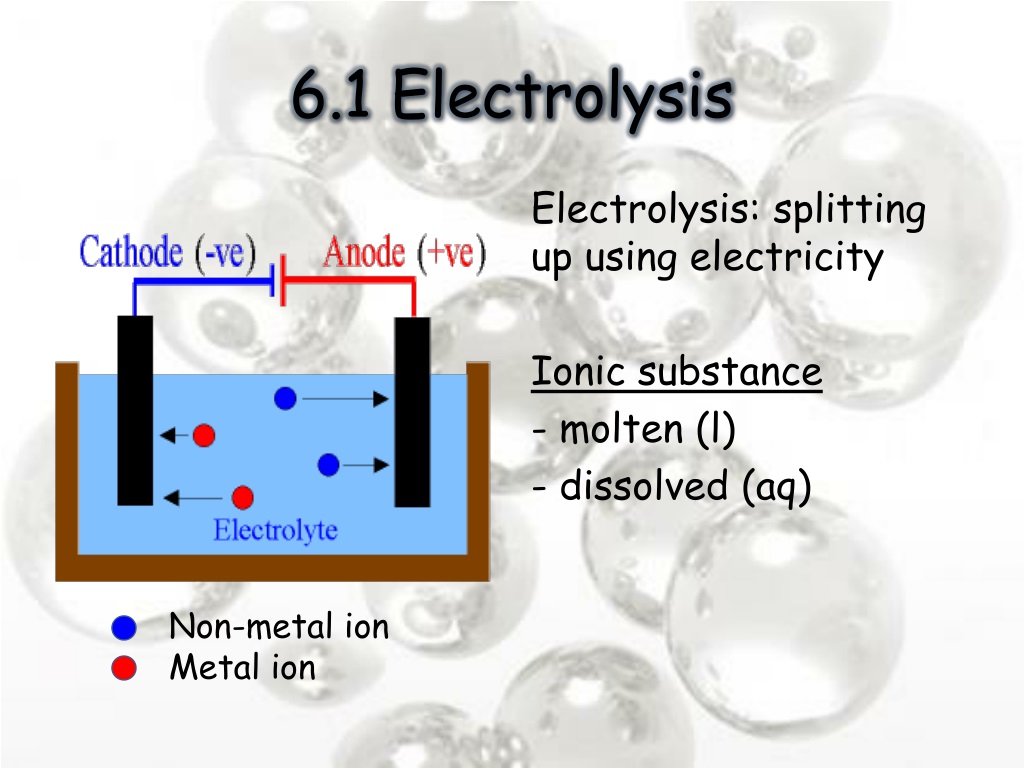
Electrode Elements Electrolysis . An external voltage is applied to drive a nonspontaneous. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. The products of electrolysis can be predicted for a given electrolyte. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. We will look at three examples of the electrolytic process, keeping. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell.
from www.slideserve.com
We will look at three examples of the electrolytic process, keeping. An external voltage is applied to drive a nonspontaneous. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis.
PPT Electrolysis PowerPoint Presentation, free download ID9353258
Electrode Elements Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. An external voltage is applied to drive a nonspontaneous. We will look at three examples of the electrolytic process, keeping. The products of electrolysis can be predicted for a given electrolyte. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. Electrolysis, process by which electric current is passed through a substance to effect a chemical change.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. We will look at three examples of the electrolytic process, keeping. Electrolysis involves using electricity to break down electrolytes to form elements. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to. Electrode Elements Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode Elements Electrolysis Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. Electrode Elements Electrolysis.
From kayleyewabarr.blogspot.com
Anode and Cathode in Electrolysis KayleyewaBarr Electrode Elements Electrolysis We will look at three examples of the electrolytic process, keeping. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. The products of electrolysis can be predicted for a given. Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis involves using. Electrode Elements Electrolysis.
From www.youtube.com
Electrolysis of Water Electrochemistry YouTube Electrode Elements Electrolysis The products of electrolysis can be predicted for a given. We will look at three examples of the electrolytic process, keeping. Electrolysis involves using electricity to break down electrolytes to form elements. An external voltage is applied to drive a nonspontaneous. The products of electrolysis can be predicted for a given electrolyte. Reactive metals are extracted from their ores using. Electrode Elements Electrolysis.
From shop.wf-education.com
Understanding Electrolysis Electrode Elements Electrolysis The products of electrolysis can be predicted for a given electrolyte. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. We will look at three examples of the electrolytic process, keeping. In an electrolytic cell, however, the opposite process, called electrolysis,. Electrode Elements Electrolysis.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Electrode Elements Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for a given electrolyte. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form elements. Think of. Electrode Elements Electrolysis.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrode Elements Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. Ionic compounds conduct electricity when molten or in solution. We will look at three examples of the electrolytic process, keeping. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to. Electrode Elements Electrolysis.
From www.researchgate.net
Schematic diagrams of an electrolysis cell for PEC CO2RR in a Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. We will look at three examples of the electrolytic process, keeping. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. An external voltage is applied to drive a nonspontaneous. Reactive metals are extracted from their ores using electrolysis. In. Electrode Elements Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID4966899 Electrode Elements Electrolysis The products of electrolysis can be predicted for a given electrolyte. Ionic compounds conduct electricity when molten or in solution. The products of electrolysis can be predicted for a given. We will look at three examples of the electrolytic process, keeping. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Think of electrolysis and electrolytic cells as the. Electrode Elements Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Electrode Elements Electrolysis The products of electrolysis can be predicted for a given. The products of electrolysis can be predicted for a given electrolyte. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. We will look at three examples of the electrolytic process, keeping. Think of electrolysis and electrolytic cells as the opposite of. Electrode Elements Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode Elements Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis. We will look. Electrode Elements Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Electrode Elements Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Reactive. Electrode Elements Electrolysis.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrode Elements Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. An external voltage is applied to drive a nonspontaneous. The products of. Electrode Elements Electrolysis.
From stoplearn.com
Electrolysis Notes 2023 Electrode Elements Electrolysis Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. We will look at three examples of the electrolytic process, keeping. In. Electrode Elements Electrolysis.
From www.alamy.com
Electrolysis of water diagram. Battery, anode, cathode, cation, anion Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form elements. We will look at three examples of the electrolytic process, keeping. Think of electrolysis and electrolytic cells as the opposite. Electrode Elements Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode Elements Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. We will look at three examples of. Electrode Elements Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrode Elements Electrolysis An external voltage is applied to drive a nonspontaneous. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. We will look at three examples of the electrolytic process, keeping. Think of electrolysis and electrolytic cells as the opposite. Electrode Elements Electrolysis.
From 2012books.lardbucket.org
Electrolysis Electrode Elements Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis involves using electricity to break down electrolytes to form elements. We will look at three examples of the electrolytic process, keeping. An external voltage is applied to drive a nonspontaneous. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores. Electrode Elements Electrolysis.
From 2012books.lardbucket.org
Electrolysis Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. We will look at three examples of the electrolytic process, keeping. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. The products of electrolysis can be predicted for a given electrolyte. Electrolysis involves using electricity to break down electrolytes to form elements. An external voltage is applied to. Electrode Elements Electrolysis.
From www.snexplores.org
Explainer What is an electrode? Electrode Elements Electrolysis Reactive metals are extracted from their ores using electrolysis. An external voltage is applied to drive a nonspontaneous. The products of electrolysis can be predicted for a given electrolyte. The products of electrolysis can be predicted for a given. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Electrolysis involves using electricity to break down electrolytes to. Electrode Elements Electrolysis.
From www.adevos.science
Electrolysis Adevoscience Electrode Elements Electrolysis We will look at three examples of the electrolytic process, keeping. Electrolysis involves using electricity to break down electrolytes to form elements. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for a given electrolyte. Ionic compounds conduct electricity when molten or. Electrode Elements Electrolysis.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Electrode Elements Electrolysis We will look at three examples of the electrolytic process, keeping. Ionic compounds conduct electricity when molten or in solution. An external voltage is applied to drive a nonspontaneous. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. The products of electrolysis can be predicted for a given electrolyte. In an electrolytic cell, however, the opposite process,. Electrode Elements Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrode Elements Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: The products of electrolysis can be predicted for a given electrolyte. Electrolysis involves using electricity to break down electrolytes to form elements. An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. We will. Electrode Elements Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. The products of electrolysis can be predicted for a given. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis involves using electricity to break down electrolytes to form elements. Reactive metals are extracted from their ores. Electrode Elements Electrolysis.
From www.vrogue.co
Schematic Diagram Of Electrolysis Process Download Sc vrogue.co Electrode Elements Electrolysis The products of electrolysis can be predicted for a given. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis, process by which electric current is. Electrode Elements Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrode Elements Electrolysis Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Electrolysis involves using electricity to break down electrolytes to form elements. Reactive metals are extracted from their ores using electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to. Electrode Elements Electrolysis.
From app.pandai.org
Electrolytic Cell Electrode Elements Electrolysis We will look at three examples of the electrolytic process, keeping. An external voltage is applied to drive a nonspontaneous. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Reactive metals are extracted from their ores using electrolysis. The products of electrolysis. Electrode Elements Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 Electrode Elements Electrolysis In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis involves using electricity to break down electrolytes to form elements. We will look at three examples of the electrolytic process, keeping. An external voltage is applied to drive a nonspontaneous. The products of electrolysis can be predicted for a given electrolyte. Ionic compounds conduct electricity when molten or. Electrode Elements Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrode Elements Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. The products of electrolysis can be predicted for a given electrolyte. Electrolysis involves using electricity to break down electrolytes to form elements. We will look at three examples of the electrolytic process, keeping. The products of electrolysis. Electrode Elements Electrolysis.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Electrode Elements Electrolysis Reactive metals are extracted from their ores using electrolysis. We will look at three examples of the electrolytic process, keeping. An external voltage is applied to drive a nonspontaneous. The products of electrolysis can be predicted for a given. Electrolysis, process by which electric current is passed through a substance to effect a chemical change. In an electrolytic cell, however,. Electrode Elements Electrolysis.
From www.knowledgeboat.com
Chapter 6 Electrolysis Selina Solutions Concise Chemistry Class 10 Electrode Elements Electrolysis Electrolysis, process by which electric current is passed through a substance to effect a chemical change. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. We will look at three examples of the electrolytic process, keeping. The products of electrolysis can be predicted for a given electrolyte. Electrolysis involves using electricity to break down electrolytes to form. Electrode Elements Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Electrode Elements Electrolysis Electrolysis involves using electricity to break down electrolytes to form elements. Think of electrolysis and electrolytic cells as the opposite of electrochemical cell. Reactive metals are extracted from their ores using electrolysis. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: An external voltage is applied to drive a nonspontaneous. We will look at three examples of the. Electrode Elements Electrolysis.
From mungfali.com
Electrolysis Process Diagram Electrode Elements Electrolysis We will look at three examples of the electrolytic process, keeping. Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given electrolyte. An external voltage is applied to drive a nonspontaneous. Think of electrolysis and electrolytic cells as the opposite. Electrode Elements Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. Electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: We will look at three examples of the electrolytic process, keeping. Electrolysis, process by which electric current is passed. Electrode Elements Electrolysis.
From studymind.co.uk
Electrolysis of Aqueous Solutions (GCSE Chemistry) Study Mind Electrode Elements Electrolysis Ionic compounds conduct electricity when molten or in solution. An external voltage is applied to drive a nonspontaneous. Reactive metals are extracted from their ores using electrolysis. We will look at three examples of the electrolytic process, keeping. The products of electrolysis can be predicted for a given. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: Electrolysis. Electrode Elements Electrolysis.