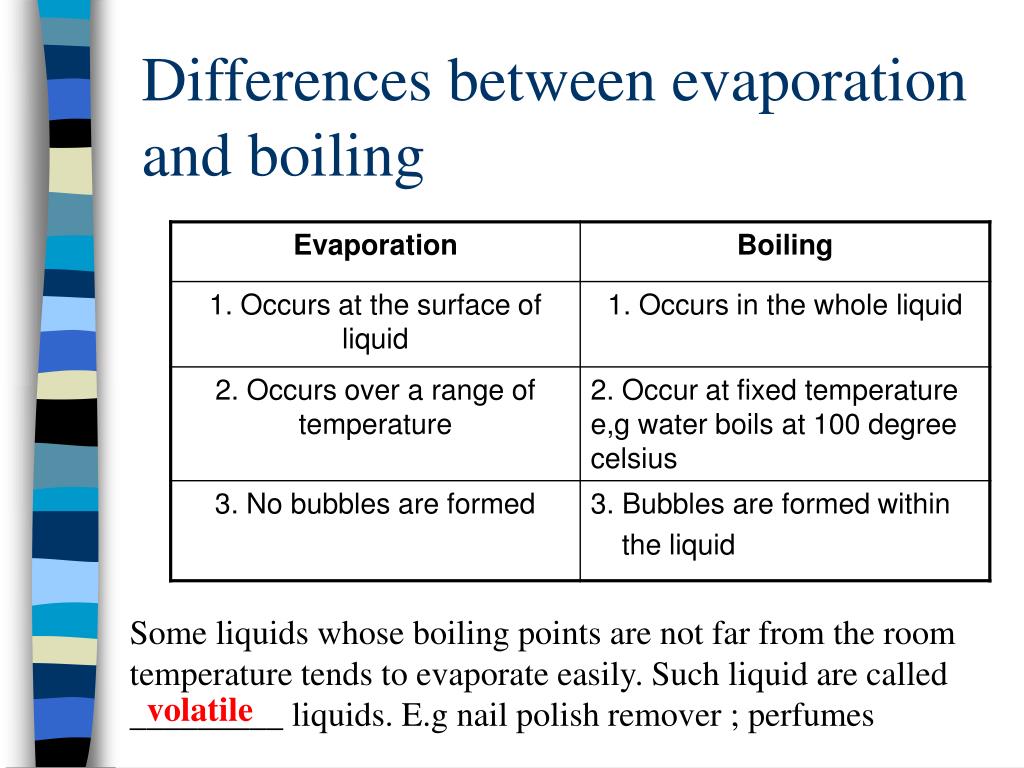
How Does Evaporation Work Below The Boiling Point . Evaporation happens when a liquid. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. The higher the temperature, the faster the evaporation. This is why water boils at 100°c at sea level—a bubble of. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. This means that at 100°c, you can have pure water vapor at atmospheric pressure. Some substances evaporate easily, some evaporate very little. It depends on the strength of the forces between particles. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler surrounding air. It is also how liquid water enters.
from www.slideserve.com
Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler surrounding air. This is why water boils at 100°c at sea level—a bubble of. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. The higher the temperature, the faster the evaporation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation happens when a liquid. It depends on the strength of the forces between particles. It is also how liquid water enters.
PPT Changes in States PowerPoint Presentation, free download ID6629377
How Does Evaporation Work Below The Boiling Point Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. This is why water boils at 100°c at sea level—a bubble of. It is also how liquid water enters. It depends on the strength of the forces between particles. Evaporation happens when a liquid. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. The higher the temperature, the faster the evaporation. Some substances evaporate easily, some evaporate very little. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler surrounding air. This means that at 100°c, you can have pure water vapor at atmospheric pressure. (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube How Does Evaporation Work Below The Boiling Point (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Some substances evaporate easily, some evaporate very little. Evaporation occurs when a liquid slowly turns into a gas below its boiling. How Does Evaporation Work Below The Boiling Point.
From sites.google.com
Y7 Science helper Reference page How Does Evaporation Work Below The Boiling Point Some substances evaporate easily, some evaporate very little. Evaporation happens when a liquid. Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler surrounding air. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Crystallisation is a separation. How Does Evaporation Work Below The Boiling Point.
From www.dreamstime.com
Evaporation and Boiling Point of Water . Vector Stock Vector How Does Evaporation Work Below The Boiling Point It depends on the strength of the forces between particles. This means that at 100°c, you can have pure water vapor at atmospheric pressure. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Crystallisation is a separation technique used to obtain crystals of a solid. How Does Evaporation Work Below The Boiling Point.
From edu.rsc.org
Evaporation explained Infographic RSC Education How Does Evaporation Work Below The Boiling Point Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Crystallisation is a separation technique used to obtain crystals of a solid solute. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The higher the temperature,. How Does Evaporation Work Below The Boiling Point.
From pediaa.com
Difference Between Evaporation and Boiling How Does Evaporation Work Below The Boiling Point Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas. How Does Evaporation Work Below The Boiling Point.
From www.youtube.com
Evaporation vs Boiling Point YouTube How Does Evaporation Work Below The Boiling Point Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. It is also how liquid water enters. The higher the temperature, the faster the evaporation. Evaporation happens when a liquid. Some substances evaporate easily,. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free How Does Evaporation Work Below The Boiling Point Crystallisation is a separation technique used to obtain crystals of a solid solute. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Evaporation, process by which an element or compound transitions from. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Changes in States PowerPoint Presentation, free download ID6629377 How Does Evaporation Work Below The Boiling Point Crystallisation is a separation technique used to obtain crystals of a solid solute. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. (a) because of the. How Does Evaporation Work Below The Boiling Point.
From www.vectorstock.com
Boiling and evaporation freezing and melting Vector Image How Does Evaporation Work Below The Boiling Point Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation happens when a liquid. This is why water boils at 100°c at sea level—a bubble of. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Liquid water is made up of. How Does Evaporation Work Below The Boiling Point.
From www.merchantnavydecoded.com
What is Evaporation and boiling? Merchant Navy Decoded How Does Evaporation Work Below The Boiling Point Some substances evaporate easily, some evaporate very little. (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Liquid water is. How Does Evaporation Work Below The Boiling Point.
From www.dreamstime.com
Evaporation and Boiling Point of Liquids Stock Illustration How Does Evaporation Work Below The Boiling Point It is also how liquid water enters. The higher the temperature, the faster the evaporation. This means that at 100°c, you can have pure water vapor at atmospheric pressure. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Evaporation happens when. How Does Evaporation Work Below The Boiling Point.
From schoolings.org
Evaporation And Boiling Meaning, Differences, Factors Affecting How Does Evaporation Work Below The Boiling Point Some substances evaporate easily, some evaporate very little. It depends on the strength of the forces between particles. This is why water boils at 100°c at sea level—a bubble of. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. (a). How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free How Does Evaporation Work Below The Boiling Point Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. This means that at 100°c, you can have pure water vapor at atmospheric pressure. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. The higher the temperature, the faster the. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID2061272 How Does Evaporation Work Below The Boiling Point This is why water boils at 100°c at sea level—a bubble of. Evaporation happens when a liquid. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. It is also how liquid water enters. This means that at 100°c, you can have pure water vapor at atmospheric pressure. Liquid water is made up of molecules of. How Does Evaporation Work Below The Boiling Point.
From www.youtube.com
phase transitions boiling evaporation condensation cooking water below How Does Evaporation Work Below The Boiling Point Evaporation happens when a liquid. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The higher the temperature, the faster the evaporation. This is why water boils at 100°c at sea level—a bubble of. (b) if the container is sealed, evaporation will continue until there is enough. How Does Evaporation Work Below The Boiling Point.
From tutors.com
Evaporation Definition & Examples (Video) How Does Evaporation Work Below The Boiling Point Evaporation happens when a liquid. Crystallisation is a separation technique used to obtain crystals of a solid solute. (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. Evaporation occurs when a liquid slowly turns into a gas below its boiling point.. How Does Evaporation Work Below The Boiling Point.
From www.toppr.com
Differences Between Evaporation and Boiling in Chemistry How Does Evaporation Work Below The Boiling Point Crystallisation is a separation technique used to obtain crystals of a solid solute. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler surrounding air. This means. How Does Evaporation Work Below The Boiling Point.
From www.slideshare.net
Evaporation How Does Evaporation Work Below The Boiling Point It depends on the strength of the forces between particles. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. This means that at 100°c, you can have pure water vapor at atmospheric pressure. This is why water boils at 100°c at sea level—a bubble of. The higher the. How Does Evaporation Work Below The Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps How Does Evaporation Work Below The Boiling Point Evaporation occurs when a liquid slowly turns into a gas below its boiling point. The higher the temperature, the faster the evaporation. It is also how liquid water enters. Some substances evaporate easily, some evaporate very little. Crystallisation is a separation technique used to obtain crystals of a solid solute. (b) if the container is sealed, evaporation will continue until. How Does Evaporation Work Below The Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point How Does Evaporation Work Below The Boiling Point Evaporation happens when a liquid. It depends on the strength of the forces between particles. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. This means that at 100°c, you can have pure water vapor at atmospheric pressure. The higher the temperature, the faster the. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Chapter 11. States of Matter PowerPoint Presentation, free How Does Evaporation Work Below The Boiling Point This is why water boils at 100°c at sea level—a bubble of. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler surrounding air. It is also. How Does Evaporation Work Below The Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts How Does Evaporation Work Below The Boiling Point Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. This is why water boils at 100°c at sea level—a bubble of. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Steam is the wet mist. How Does Evaporation Work Below The Boiling Point.
From slideplayer.com
Phases and Changes in Matter ppt download How Does Evaporation Work Below The Boiling Point Evaporation happens when a liquid. It depends on the strength of the forces between particles. Some substances evaporate easily, some evaporate very little. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. This is why water boils at 100°c at sea level—a bubble of. Crystallisation is a separation. How Does Evaporation Work Below The Boiling Point.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 How Does Evaporation Work Below The Boiling Point Evaporation happens when a liquid. This is why water boils at 100°c at sea level—a bubble of. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a solid solute. Some substances evaporate easily, some evaporate very little. It depends on the strength of the forces. How Does Evaporation Work Below The Boiling Point.
From slideplayer.com
Phases and Heat Chapters 13 & ppt download How Does Evaporation Work Below The Boiling Point (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Some substances evaporate easily, some evaporate very little. It depends on the strength of the forces between particles. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below. How Does Evaporation Work Below The Boiling Point.
From www.youtube.com
How Clothes get dry up below Boiling Point of Water/Evaporation/Boiling How Does Evaporation Work Below The Boiling Point Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Some substances evaporate easily, some evaporate very little. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. The higher the temperature, the faster the evaporation. (b) if the container is sealed, evaporation. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free How Does Evaporation Work Below The Boiling Point Some substances evaporate easily, some evaporate very little. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. It depends on the strength of the forces between particles. The higher the temperature, the faster the evaporation. This is why water boils. How Does Evaporation Work Below The Boiling Point.
From www.youtube.com
Difference between Evaporation and Boiling science learning academy How Does Evaporation Work Below The Boiling Point It is also how liquid water enters. Crystallisation is a separation technique used to obtain crystals of a solid solute. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Liquid water. How Does Evaporation Work Below The Boiling Point.
From www.bbc.co.uk
Evaporation BBC Bitesize How Does Evaporation Work Below The Boiling Point Crystallisation is a separation technique used to obtain crystals of a solid solute. It depends on the strength of the forces between particles. (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Evaporation, process by which an element or compound transitions from its liquid state. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download How Does Evaporation Work Below The Boiling Point (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. The higher the temperature, the faster the evaporation. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation happens when a liquid. It depends on the strength. How Does Evaporation Work Below The Boiling Point.
From www.diffzy.com
Evaporation vs Boiling What's the Difference (With Table) How Does Evaporation Work Below The Boiling Point (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Evaporation happens when a liquid. It is also how liquid water enters. (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below. How Does Evaporation Work Below The Boiling Point.
From slideplayer.com
Phases and Behavior of Matter ppt download How Does Evaporation Work Below The Boiling Point (b) if the container is sealed, evaporation will continue until there is enough vapor density for the condensation rate to equal the evaporation rate. Some substances evaporate easily, some evaporate very little. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. (a) because of the distribution of speeds and kinetic energies, some water molecules can. How Does Evaporation Work Below The Boiling Point.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation How Does Evaporation Work Below The Boiling Point Evaporation happens when a liquid. The higher the temperature, the faster the evaporation. (a) because of the distribution of speeds and kinetic energies, some water molecules can break away to the vapor phase even at temperatures below the ordinary boiling point. Steam is the wet mist that forms above boiling water as the hot, invisible water vapor mixes with cooler. How Does Evaporation Work Below The Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free How Does Evaporation Work Below The Boiling Point It depends on the strength of the forces between particles. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. (a) because of the distribution of speeds and. How Does Evaporation Work Below The Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How Does Evaporation Work Below The Boiling Point Some substances evaporate easily, some evaporate very little. Crystallisation is a separation technique used to obtain crystals of a solid solute. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation can happen at any temperature, unlike boiling, which only happens at the boiling point. Evaporation, process by which an element or compound transitions from. How Does Evaporation Work Below The Boiling Point.