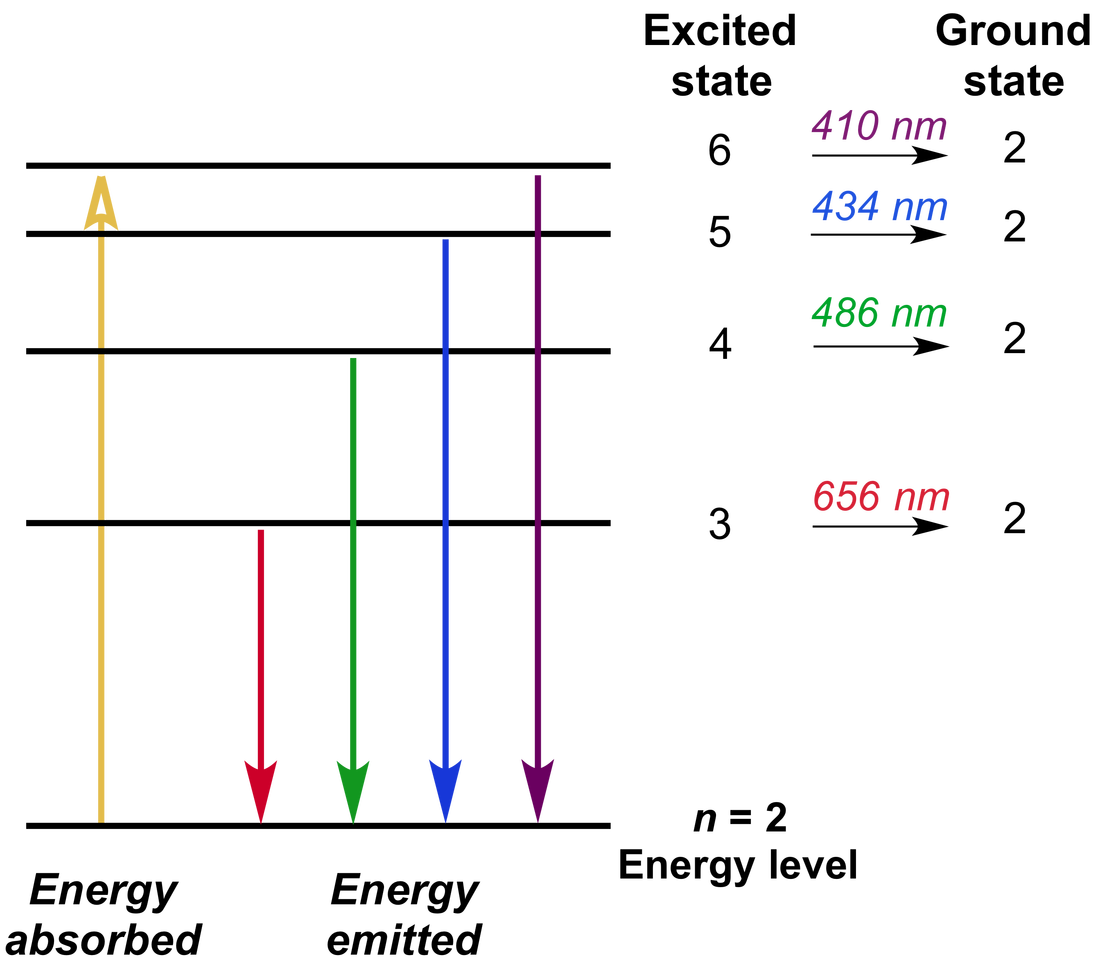
Emission Spectra Hydrogen . To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. Nist atomic spectra database [1], gfall.dat file from. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. Figure 7.3.5 the emission spectra of elements compared with hydrogen. These images show (a) hydrogen gas, which is atomized to hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. It also looks at how the spectrum. This suggests that electrons can. Development of current atomic theory. Each line is a specific energy value. The three atomic emission spectra for hydrogen can be shown on graph bellow: The line emission (visible) spectrum of hydrogen.
from chemistrypuns-periodically.weebly.com
These images show (a) hydrogen gas, which is atomized to hydrogen. The three atomic emission spectra for hydrogen can be shown on graph bellow: Development of current atomic theory. This suggests that electrons can. The line emission (visible) spectrum of hydrogen. It also looks at how the spectrum. Figure 7.3.5 the emission spectra of elements compared with hydrogen. Each line is a specific energy value. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. Nist atomic spectra database [1], gfall.dat file from.
Chemistry Electron Emission Spectrum
Emission Spectra Hydrogen This suggests that electrons can. This suggests that electrons can. Figure 7.3.5 the emission spectra of elements compared with hydrogen. These images show (a) hydrogen gas, which is atomized to hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. The three atomic emission spectra for hydrogen can be shown on graph bellow: The line emission (visible) spectrum of hydrogen. Each line is a specific energy value. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. It also looks at how the spectrum. Nist atomic spectra database [1], gfall.dat file from. Development of current atomic theory. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom.
From gerteditor.weebly.com
Atomic emission spectrum of hydrogen gerteditor Emission Spectra Hydrogen Development of current atomic theory. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. Each line is a specific energy value. This suggests that electrons can. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the. Emission Spectra Hydrogen.
From mavink.com
Visible Line Spectrum Of Hydrogen Emission Spectra Hydrogen Development of current atomic theory. Each line is a specific energy value. The three atomic emission spectra for hydrogen can be shown on graph bellow: This suggests that electrons can. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. When an electric current is passed through a glass. Emission Spectra Hydrogen.
From hydrogenpotanezu.blogspot.com
Hydrogen Hydrogen Line Spectra Emission Spectra Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. These images show (a) hydrogen gas, which is atomized to hydrogen. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. The three atomic emission spectra. Emission Spectra Hydrogen.
From pixels.com
Emission Spectrum Of Hydrogen Photograph by Dept. Of Physics, Imperial Emission Spectra Hydrogen These images show (a) hydrogen gas, which is atomized to hydrogen. Nist atomic spectra database [1], gfall.dat file from. The three atomic emission spectra for hydrogen can be shown on graph bellow: It also looks at how the spectrum. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of. Emission Spectra Hydrogen.
From www.dreamstime.com
Spectrum. Spectral Line for Example Hydrogen. Emission Lines and Emission Spectra Hydrogen The line emission (visible) spectrum of hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. The three atomic emission spectra for hydrogen can be shown on graph bellow: This suggests that electrons can. These images show (a) hydrogen gas, which is atomized to hydrogen. Figure 7.3.5 the. Emission Spectra Hydrogen.
From sciencephotogallery.com
Hydrogen Emission And Absorption Spectra by Science Photo Library Emission Spectra Hydrogen To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. These images show (a) hydrogen gas, which is atomized to hydrogen. The line emission (visible) spectrum of hydrogen. Each line is a specific energy value. Development of current atomic theory. Nist atomic spectra database [1], gfall.dat file. Emission Spectra Hydrogen.
From www.sciencephoto.com
Hydrogen emission and absorption spectra Stock Image C025/8082 Emission Spectra Hydrogen This suggests that electrons can. These images show (a) hydrogen gas, which is atomized to hydrogen. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. Nist atomic spectra database [1], gfall.dat file from. It also looks at how the spectrum. Each line is a specific energy. Emission Spectra Hydrogen.
From mavink.com
Absorption Spectrum Of Hydrogen Emission Spectra Hydrogen Nist atomic spectra database [1], gfall.dat file from. It also looks at how the spectrum. Figure 7.3.5 the emission spectra of elements compared with hydrogen. This suggests that electrons can. The line emission (visible) spectrum of hydrogen. These images show (a) hydrogen gas, which is atomized to hydrogen. To introduce the concept of absorption and emission line spectra and describe. Emission Spectra Hydrogen.
From hydrogenpotanezu.blogspot.com
Hydrogen Hydrogen Spectrum Emission Spectra Hydrogen To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. Each line is a specific energy value. This suggests that electrons can. These images show (a) hydrogen gas, which is atomized to hydrogen. The line emission (visible) spectrum of hydrogen. When an electric current is passed through. Emission Spectra Hydrogen.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Hydrogen This suggests that electrons can. The three atomic emission spectra for hydrogen can be shown on graph bellow: It also looks at how the spectrum. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements. Emission Spectra Hydrogen.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube Emission Spectra Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. Figure 7.3.5 the emission spectra of elements compared with hydrogen. The line emission (visible) spectrum of hydrogen. Nist atomic spectra database [1], gfall.dat file from. These images show (a) hydrogen gas, which is atomized to hydrogen. This page introduces the. Emission Spectra Hydrogen.
From mungfali.com
Hydrogen Atomic Emission Spectrum Emission Spectra Hydrogen To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. Nist atomic spectra database [1], gfall.dat file from. It also looks at how the spectrum. These images. Emission Spectra Hydrogen.
From www.sciencephoto.com
Emission spectrum of hydrogen Stock Image A150/0020 Science Photo Emission Spectra Hydrogen The line emission (visible) spectrum of hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. This suggests that electrons can. Figure 7.3.5 the emission spectra of elements compared with hydrogen. Each line is a specific energy value. These images show (a) hydrogen gas, which is atomized to. Emission Spectra Hydrogen.
From byjus.com
Hydrogen Spectrum Balmer Series, Definition, Diagram, Spectrum Emission Spectra Hydrogen Nist atomic spectra database [1], gfall.dat file from. Development of current atomic theory. It also looks at how the spectrum. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. This suggests that electrons can. The three atomic emission spectra for hydrogen can be shown on graph bellow: This page. Emission Spectra Hydrogen.
From animalia-life.club
Bohr Model Of Hydrogen Emission Spectrum Emission Spectra Hydrogen Nist atomic spectra database [1], gfall.dat file from. The three atomic emission spectra for hydrogen can be shown on graph bellow: The line emission (visible) spectrum of hydrogen. It also looks at how the spectrum. Figure 7.3.5 the emission spectra of elements compared with hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements. Emission Spectra Hydrogen.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectra Hydrogen These images show (a) hydrogen gas, which is atomized to hydrogen. Each line is a specific energy value. It also looks at how the spectrum. Nist atomic spectra database [1], gfall.dat file from. The line emission (visible) spectrum of hydrogen. This suggests that electrons can. To introduce the concept of absorption and emission line spectra and describe the balmer equation. Emission Spectra Hydrogen.
From mungfali.com
The Hydrogen Spectrum Emission Spectra Hydrogen These images show (a) hydrogen gas, which is atomized to hydrogen. The line emission (visible) spectrum of hydrogen. Development of current atomic theory. Figure 7.3.5 the emission spectra of elements compared with hydrogen. Nist atomic spectra database [1], gfall.dat file from. This suggests that electrons can. The three atomic emission spectra for hydrogen can be shown on graph bellow: Each. Emission Spectra Hydrogen.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Emission Spectra Hydrogen It also looks at how the spectrum. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. The three atomic emission spectra for hydrogen can be shown on graph bellow: To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines. Emission Spectra Hydrogen.
From chelsea-well-johnston.blogspot.com
Describe the Emission Spectrum of Hydrogen Emission Spectra Hydrogen Each line is a specific energy value. These images show (a) hydrogen gas, which is atomized to hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. The three atomic emission spectra for hydrogen can be shown on graph bellow: This page introduces the atomic hydrogen emission spectrum, showing. Emission Spectra Hydrogen.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Emission Spectra Hydrogen Figure 7.3.5 the emission spectra of elements compared with hydrogen. Development of current atomic theory. It also looks at how the spectrum. Each line is a specific energy value. This suggests that electrons can. The line emission (visible) spectrum of hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within. Emission Spectra Hydrogen.
From kerywatches.weebly.com
Atomic emission spectrum of hydrogen kerywatches Emission Spectra Hydrogen It also looks at how the spectrum. The line emission (visible) spectrum of hydrogen. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. This suggests that electrons can. Nist atomic spectra database [1], gfall.dat file from. These images show (a) hydrogen gas, which is atomized to. Emission Spectra Hydrogen.
From mungfali.com
Hydrogen Atomic Emission Spectrum Emission Spectra Hydrogen Nist atomic spectra database [1], gfall.dat file from. These images show (a) hydrogen gas, which is atomized to hydrogen. Development of current atomic theory. The three atomic emission spectra for hydrogen can be shown on graph bellow: This suggests that electrons can. It also looks at how the spectrum. To introduce the concept of absorption and emission line spectra and. Emission Spectra Hydrogen.
From explorerlasi.weebly.com
Hydrogen line emission spectrum explorerLasi Emission Spectra Hydrogen To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. Each line is a specific energy value. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. The three atomic emission spectra for hydrogen can be shown. Emission Spectra Hydrogen.
From chemwiki.ucdavis.edu
The Bohr Atom Chemwiki Emission Spectra Hydrogen To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines of atomic hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. These images show (a) hydrogen gas, which is atomized to hydrogen. This suggests that electrons can. Development. Emission Spectra Hydrogen.
From mavink.com
Visible Line Spectrum Of Hydrogen Emission Spectra Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. Each line is a specific energy value. Nist atomic spectra database [1], gfall.dat file from. This suggests that electrons can. These images show (a) hydrogen gas, which is atomized to hydrogen. The three atomic emission spectra for hydrogen can. Emission Spectra Hydrogen.
From sciencephotogallery.com
Hydrogen And Helium Spectra by Science Photo Library Emission Spectra Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. Development of current atomic theory. These images show (a) hydrogen gas, which is atomized to hydrogen. The line emission (visible) spectrum of hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy. Emission Spectra Hydrogen.
From chem.libretexts.org
1.4 The Hydrogen Atomic Spectrum Chemistry LibreTexts Emission Spectra Hydrogen Development of current atomic theory. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. The three atomic emission spectra for hydrogen can be shown on graph bellow: The line emission (visible) spectrum of hydrogen. Figure 7.3.5 the emission spectra of elements compared with hydrogen. Each line is a. Emission Spectra Hydrogen.
From www.youtube.com
03.03 The Emission Spectrum of the Hydrogen Atom YouTube Emission Spectra Hydrogen Development of current atomic theory. Figure 7.3.5 the emission spectra of elements compared with hydrogen. Each line is a specific energy value. These images show (a) hydrogen gas, which is atomized to hydrogen. It also looks at how the spectrum. To introduce the concept of absorption and emission line spectra and describe the balmer equation to describe the visible lines. Emission Spectra Hydrogen.
From www.vrogue.co
Atomic Spectrum Of Hydrogen In 2021 Hydrogen Atom Ato vrogue.co Emission Spectra Hydrogen When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. The line emission (visible) spectrum of hydrogen. Each line is a specific energy value. This suggests that electrons can.. Emission Spectra Hydrogen.
From emiliano-blogmassey.blogspot.com
Describe the Emission Spectrum of Hydrogen Ib Chemistry Emission Spectra Hydrogen This suggests that electrons can. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. The three atomic emission spectra for hydrogen can be shown on graph bellow: These images show (a) hydrogen gas, which is atomized to hydrogen. When an electric current is passed through a glass tube. Emission Spectra Hydrogen.
From www.alamy.com
Diagram showing the absorption and emission spectra of the elements Emission Spectra Hydrogen It also looks at how the spectrum. Nist atomic spectra database [1], gfall.dat file from. These images show (a) hydrogen gas, which is atomized to hydrogen. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. The three atomic emission spectra for hydrogen can be shown on graph bellow:. Emission Spectra Hydrogen.
From ece.umd.edu
Hydrogen atom, spectrum and electron transitions Emission Spectra Hydrogen Each line is a specific energy value. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives. The line emission (visible) spectrum of hydrogen. Nist atomic spectra database [1], gfall.dat file from. It also looks at how the spectrum. Development of current atomic theory. This page introduces the atomic hydrogen. Emission Spectra Hydrogen.
From cowboywas.weebly.com
Line emission spectrum of hydrogen cowboyWas Emission Spectra Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. These images show (a) hydrogen gas, which is atomized to hydrogen. The three atomic emission spectra for hydrogen can be shown on graph bellow: The line emission (visible) spectrum of hydrogen. It also looks at how the spectrum. Figure. Emission Spectra Hydrogen.
From gerteditor.weebly.com
Atomic emission spectrum of hydrogen gerteditor Emission Spectra Hydrogen This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. This suggests that electrons can. The three atomic emission spectra for hydrogen can be shown on graph bellow: It also looks at how the spectrum. To introduce the concept of absorption and emission line spectra and describe the balmer. Emission Spectra Hydrogen.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Hydrogen The line emission (visible) spectrum of hydrogen. Development of current atomic theory. It also looks at how the spectrum. This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. Nist atomic spectra database [1], gfall.dat file from. Figure 7.3.5 the emission spectra of elements compared with hydrogen. To introduce. Emission Spectra Hydrogen.