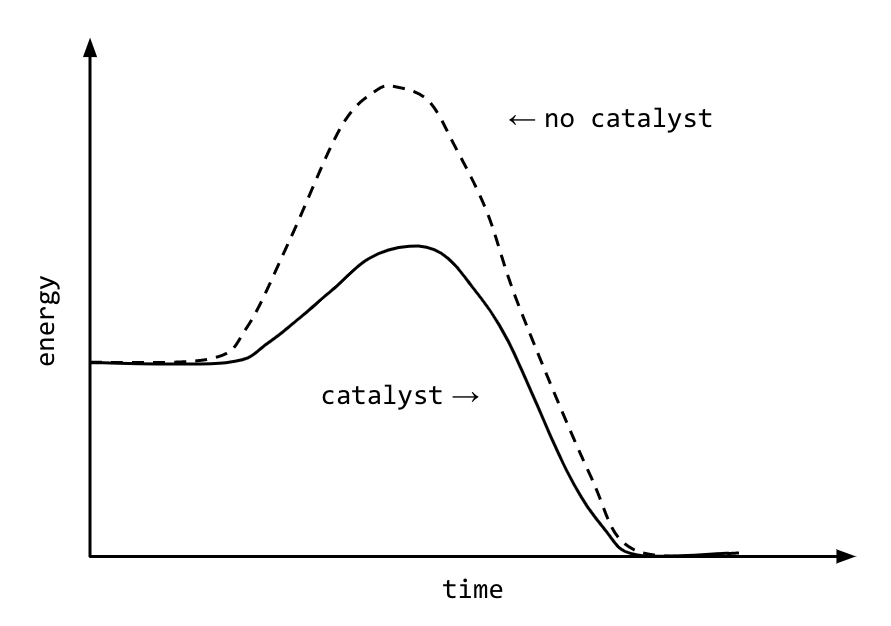
Do Catalysts Increase Activation Energy . A catalyst provides an alternative route for the reaction with a lower activation energy. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst provides an alternative route for the. A catalyst is not destroyed or. the activation energy of a reaction is 19.0 kj/mol. adding a catalyst has exactly this effect of shifting the activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. adding a catalyst has this effect on activation energy. Catalysts are defined as substances. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. When a catalyst is used at 300 k, the rate increases one.
from nesslabs.com
Catalysts are defined as substances. A catalyst lowers the activation energy of a chemical reaction. adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst is not destroyed or. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst provides an alternative route for the. the activation energy of a reaction is 19.0 kj/mol. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. adding a catalyst has this effect on activation energy.
Activation energy the chemistry of getting started Ness Labs
Do Catalysts Increase Activation Energy effect of enzymes and catalysts. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst is not destroyed or. A catalyst provides an alternative route for the. Catalysts are defined as substances. When a catalyst is used at 300 k, the rate increases one. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst provides an alternative route for the reaction with a lower activation energy. the activation energy of a reaction is 19.0 kj/mol. adding a catalyst has this effect on activation energy. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. adding a catalyst has exactly this effect of shifting the activation energy. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be.
From slideplayer.com
Biochemistry 412 Enzyme I March 29th, ppt download Do Catalysts Increase Activation Energy When a catalyst is used at 300 k, the rate increases one. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst lowers the activation energy of a chemical reaction. A catalyst is not destroyed or.. Do Catalysts Increase Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Do Catalysts Increase Activation Energy effect of enzymes and catalysts. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. Catalysts are defined as substances. A catalyst is not destroyed or. adding a catalyst has exactly this effect of shifting the activation energy. catalysts are substances that increase reaction rate by lowering. Do Catalysts Increase Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Do Catalysts Increase Activation Energy effect of enzymes and catalysts. When a catalyst is used at 300 k, the rate increases one. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst lowers the activation energy of a chemical reaction. Catalysts are defined as substances. catalysts provide a means of reducing. Do Catalysts Increase Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Do Catalysts Increase Activation Energy catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts are defined as substances. When a catalyst is used at 300 k, the rate increases one. adding a catalyst has this effect on activation energy. the activation energy of a reaction is 19.0 kj/mol. A catalyst provides an alternative route for the reaction with. Do Catalysts Increase Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Do Catalysts Increase Activation Energy effect of enzymes and catalysts. Catalysts are defined as substances. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst lowers the activation energy of a chemical reaction. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur.. Do Catalysts Increase Activation Energy.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog Do Catalysts Increase Activation Energy A catalyst is not destroyed or. When a catalyst is used at 300 k, the rate increases one. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. adding a catalyst has this effect on activation energy.. Do Catalysts Increase Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Do Catalysts Increase Activation Energy catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst provides an alternative route for the. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst lowers the activation energy of a chemical reaction. A catalyst is not destroyed or. When a catalyst. Do Catalysts Increase Activation Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Do Catalysts Increase Activation Energy Catalysts are defined as substances. A catalyst provides an alternative route for the reaction with a lower activation energy. When a catalyst is used at 300 k, the rate increases one. adding a catalyst has exactly this effect of shifting the activation energy. effect of enzymes and catalysts. A catalyst is not destroyed or. catalysts are substances. Do Catalysts Increase Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Do Catalysts Increase Activation Energy When a catalyst is used at 300 k, the rate increases one. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst provides an alternative route for the reaction with a lower activation energy. adding a catalyst has this effect on activation energy. the activation energy. Do Catalysts Increase Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Do Catalysts Increase Activation Energy A catalyst provides an alternative route for the. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. When a catalyst is used at 300 k, the rate increases one. effect of enzymes and catalysts. adding a catalyst has exactly this effect of shifting the activation energy. A catalyst. Do Catalysts Increase Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Do Catalysts Increase Activation Energy Catalysts are defined as substances. A catalyst lowers the activation energy of a chemical reaction. A catalyst provides an alternative route for the. adding a catalyst has exactly this effect of shifting the activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. When a catalyst is used at 300 k, the rate increases. Do Catalysts Increase Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Do Catalysts Increase Activation Energy effect of enzymes and catalysts. A catalyst provides an alternative route for the. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst is not destroyed or. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A. Do Catalysts Increase Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Do Catalysts Increase Activation Energy A catalyst provides an alternative route for the reaction with a lower activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. When a catalyst is used at 300 k, the rate increases one. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A. Do Catalysts Increase Activation Energy.
From slideplayer.com
Chemical PE Curves Mr. Shields Regents Chemistry U13 L ppt Do Catalysts Increase Activation Energy A catalyst provides an alternative route for the reaction with a lower activation energy. adding a catalyst has exactly this effect of shifting the activation energy. the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one. catalysts provide a means of reducing \(e_a\) and increasing the. Do Catalysts Increase Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Do Catalysts Increase Activation Energy catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst lowers the activation energy of a chemical reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. adding a catalyst has this effect on activation energy. catalysts are substances that increase. Do Catalysts Increase Activation Energy.
From circuitwiringtray.z13.web.core.windows.net
Diagram Of Activation Energy Do Catalysts Increase Activation Energy When a catalyst is used at 300 k, the rate increases one. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst lowers the activation energy of a chemical reaction. A catalyst provides an alternative route for the. A catalyst provides an alternative route for the reaction with a lower activation energy. effect of. Do Catalysts Increase Activation Energy.
From slideplayer.com
A catalyst lowers activation energy. ppt download Do Catalysts Increase Activation Energy adding a catalyst has this effect on activation energy. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst is not destroyed or. adding a catalyst has. Do Catalysts Increase Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Do Catalysts Increase Activation Energy Catalysts are defined as substances. adding a catalyst has this effect on activation energy. effect of enzymes and catalysts. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be.. Do Catalysts Increase Activation Energy.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Do Catalysts Increase Activation Energy Catalysts are defined as substances. adding a catalyst has exactly this effect of shifting the activation energy. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst provides an alternative route for the reaction. Do Catalysts Increase Activation Energy.
From www.chim.lu
Activation energy and catalysis Do Catalysts Increase Activation Energy adding a catalyst has this effect on activation energy. Catalysts are defined as substances. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst is not destroyed or. effect of enzymes and catalysts. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. adding a catalyst has exactly. Do Catalysts Increase Activation Energy.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Do Catalysts Increase Activation Energy catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst provides an alternative route for the. Catalysts are defined as substances. the activation energy of a reaction is 19.0 kj/mol. effect of enzymes and catalysts. adding a catalyst has this effect on activation energy. A. Do Catalysts Increase Activation Energy.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at Do Catalysts Increase Activation Energy effect of enzymes and catalysts. A catalyst provides an alternative route for the. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. adding a catalyst has this effect on activation energy. When a catalyst is used at 300 k, the rate increases one. A catalyst is not. Do Catalysts Increase Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Do Catalysts Increase Activation Energy catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. A catalyst lowers the activation energy of a chemical reaction. adding a catalyst has this effect on activation energy. effect of enzymes and catalysts. adding a catalyst has exactly this effect of shifting the activation energy. . Do Catalysts Increase Activation Energy.
From www.chegg.com
Solved How do catalysts increase the rate of a reaction? Do Catalysts Increase Activation Energy Catalysts are defined as substances. When a catalyst is used at 300 k, the rate increases one. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. the activation energy of a reaction is 19.0 kj/mol. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A. Do Catalysts Increase Activation Energy.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Do Catalysts Increase Activation Energy A catalyst provides an alternative route for the reaction with a lower activation energy. effect of enzymes and catalysts. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. Catalysts are defined as substances. When a. Do Catalysts Increase Activation Energy.
From 2012books.lardbucket.org
Catalysis Do Catalysts Increase Activation Energy the activation energy of a reaction is 19.0 kj/mol. A catalyst provides an alternative route for the. Catalysts are defined as substances. A catalyst is not destroyed or. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. catalysts provide a means of reducing \(e_a\) and increasing the reaction. Do Catalysts Increase Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Do Catalysts Increase Activation Energy When a catalyst is used at 300 k, the rate increases one. the activation energy of a reaction is 19.0 kj/mol. adding a catalyst has exactly this effect of shifting the activation energy. A catalyst provides an alternative route for the. Catalysts are defined as substances. catalysts provide a means of reducing \(e_a\) and increasing the reaction. Do Catalysts Increase Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Do Catalysts Increase Activation Energy A catalyst is not destroyed or. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. Catalysts are defined as substances. When a catalyst is used at 300 k, the rate increases one. A catalyst provides an alternative route for the. the activation energy of a reaction is 19.0 kj/mol.. Do Catalysts Increase Activation Energy.
From www.pnnl.gov
Catalysis PNNL Do Catalysts Increase Activation Energy When a catalyst is used at 300 k, the rate increases one. A catalyst lowers the activation energy of a chemical reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. effect of enzymes and catalysts. Catalysts are defined as substances. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate.. Do Catalysts Increase Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Do Catalysts Increase Activation Energy effect of enzymes and catalysts. A catalyst is not destroyed or. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst provides an alternative route for the reaction with a lower activation energy. A catalyst provides an alternative route for the. catalysts make this process more efficient. Do Catalysts Increase Activation Energy.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Do Catalysts Increase Activation Energy A catalyst provides an alternative route for the. catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. adding a catalyst. Do Catalysts Increase Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Do Catalysts Increase Activation Energy Catalysts are defined as substances. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. adding a catalyst has this effect on activation energy. A catalyst is not destroyed or. adding a catalyst has exactly this effect of shifting the activation energy. effect of enzymes and catalysts. When a catalyst is used at 300. Do Catalysts Increase Activation Energy.
From byjus.com
How does catalyst affect activation energy? Do Catalysts Increase Activation Energy Catalysts are defined as substances. A catalyst is not destroyed or. effect of enzymes and catalysts. adding a catalyst has exactly this effect of shifting the activation energy. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. adding a catalyst has this effect on activation energy.. Do Catalysts Increase Activation Energy.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Do Catalysts Increase Activation Energy the activation energy of a reaction is 19.0 kj/mol. When a catalyst is used at 300 k, the rate increases one. A catalyst is not destroyed or. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. adding a catalyst has exactly this effect of shifting the activation. Do Catalysts Increase Activation Energy.
From slideplayer.com
Lecture 1405 Reaction Mechanism and Catalysis ppt download Do Catalysts Increase Activation Energy the activation energy of a reaction is 19.0 kj/mol. A catalyst provides an alternative route for the. A catalyst provides an alternative route for the reaction with a lower activation energy. effect of enzymes and catalysts. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts are defined as substances. catalysts are substances. Do Catalysts Increase Activation Energy.