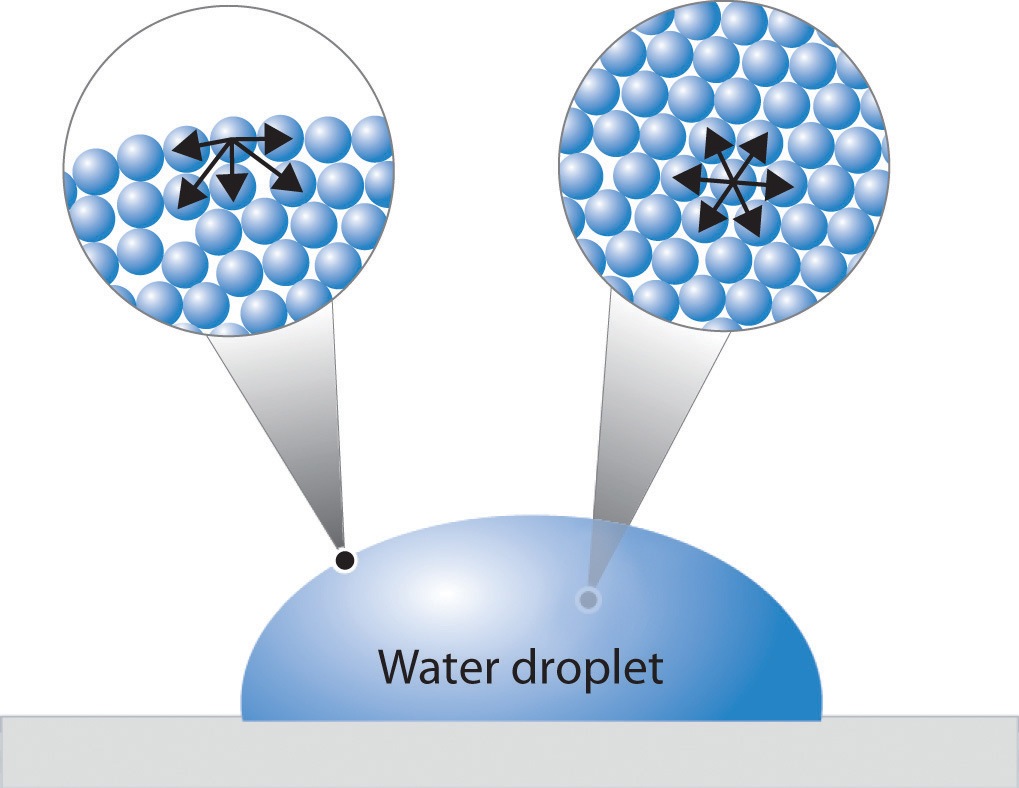
How Much Is Surface Tension Of Water . Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Water has a surface tension of 0.07275. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Since these intermolecular forces vary depending on the nature of the liquid (e.… It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. A fluid surface has a. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a manifestation of.
from chem.libretexts.org
Surface tension is a manifestation of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces vary depending on the nature of the liquid (e.… Water has a surface tension of 0.07275. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. A fluid surface has a. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Surface Tension Chemistry LibreTexts
How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a manifestation of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275. Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. A fluid surface has a. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID How Much Is Surface Tension Of Water It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. A fluid surface has. How Much Is Surface Tension Of Water.
From www.moleaer.com
How Nanobubble Technology Helps Growers with Sustainable Agriculture How Much Is Surface Tension Of Water It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. A fluid surface has a. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension. How Much Is Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension. How Much Is Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Much Is Surface Tension Of Water Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface. How Much Is Surface Tension Of Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the elastic tendency of fluid surfaces. How Much Is Surface Tension Of Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Much Is Surface Tension Of Water It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a physical. How Much Is Surface Tension Of Water.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation How Much Is Surface Tension Of Water Water has a surface tension of 0.07275. Surface tension is a manifestation of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a physical property defined as the amount of force. How Much Is Surface Tension Of Water.
From ar.inspiredpencil.com
Surface Tension Water How Much Is Surface Tension Of Water Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a. How Much Is Surface Tension Of Water.
From printableangliavone.z21.web.core.windows.net
Give An Example Of Surface Tension How Much Is Surface Tension Of Water Surface tension is a manifestation of. A fluid surface has a. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Water has a surface tension of 0.07275.. How Much Is Surface Tension Of Water.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1714027 How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Next to mercury, water has the highest surface tension of all commonly occurring liquids. A fluid surface has a. Water has a surface tension of. How Much Is Surface Tension Of Water.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Surface tension. How Much Is Surface Tension Of Water.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a surface tension of 0.07275. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. A fluid surface. How Much Is Surface Tension Of Water.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Since. How Much Is Surface Tension Of Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). A fluid surface has a. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Since these intermolecular forces vary depending on the nature of. How Much Is Surface Tension Of Water.
From www.youtube.com
Surface Tension of Water Explained YouTube How Much Is Surface Tension Of Water Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension may be expressed, therefore, in units of energy per unit area (square metres). It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Next to mercury, water has the highest surface tension of. How Much Is Surface Tension Of Water.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Much Is Surface Tension Of Water Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces vary depending on the nature of the liquid (e.… Water has a surface tension of 0.07275. A fluid surface has a. Surface tension is a manifestation. How Much Is Surface Tension Of Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a manifestation of. A fluid surface has a. Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next. How Much Is Surface Tension Of Water.
From www.studypool.com
SOLUTION Surface tension of milk Studypool How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Surface tension is a manifestation of. A fluid surface has a. Surface tension is the elastic tendency of. How Much Is Surface Tension Of Water.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Much Is Surface Tension Of Water Water has a surface tension of 0.07275. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. It seems. How Much Is Surface Tension Of Water.
From chart-studio.plotly.com
The Effect of pH on the Surface Tension of Water bar chart made by How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275. Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is a manifestation of. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on. How Much Is Surface Tension Of Water.
From ar.inspiredpencil.com
Surface Tension Of Water On A Penny How Much Is Surface Tension Of Water Water has a surface tension of 0.07275. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Since these intermolecular forces vary depending on the nature of the liquid (e.… A fluid. How Much Is Surface Tension Of Water.
From www.youtube.com
Walking on Water!! Surface Tension YouTube How Much Is Surface Tension Of Water A fluid surface has a. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is a manifestation of. Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is the energy, or work, required to increase the surface area. How Much Is Surface Tension Of Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? How Much Is Surface Tension Of Water Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since. How Much Is Surface Tension Of Water.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Much Is Surface Tension Of Water Surface tension is a manifestation of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Water has a surface tension of 0.07275. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Since these. How Much Is Surface Tension Of Water.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download How Much Is Surface Tension Of Water Surface tension is a manifestation of. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid.. How Much Is Surface Tension Of Water.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.… Next to mercury, water has the highest surface tension of all commonly occurring liquids. Water has a surface tension of 0.07275. Surface tension is a physical property. How Much Is Surface Tension Of Water.
From www.youtube.com
What is surface tension of water explained in detail? YouTube How Much Is Surface Tension Of Water Water has a surface tension of 0.07275. Surface tension is a manifestation of. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.…. How Much Is Surface Tension Of Water.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Much Is Surface Tension Of Water Surface tension is a manifestation of. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Water has a surface tension of 0.07275. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Since these intermolecular forces vary depending on the. How Much Is Surface Tension Of Water.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules How Much Is Surface Tension Of Water Surface tension is a manifestation of. Surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. Water has a surface tension of 0.07275. Since these intermolecular forces vary depending on the nature of the liquid (e.… Next to mercury, water has the highest surface tension of all commonly occurring liquids. A fluid surface has. How Much Is Surface Tension Of Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Much Is Surface Tension Of Water Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. A fluid surface has a. Surface tension is the elastic tendency of fluid surfaces that lets insects to. How Much Is Surface Tension Of Water.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Much Is Surface Tension Of Water Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension may be expressed,. How Much Is Surface Tension Of Water.
From thehomeschoolscientist.com
Surface Tension In Water Explanation and Experiment The Homeschool How Much Is Surface Tension Of Water Water has a surface tension of 0.07275. Since these intermolecular forces vary depending on the nature of the liquid (e.… Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a physical property. How Much Is Surface Tension Of Water.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a. Surface tension is. How Much Is Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How Much Is Surface Tension Of Water It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension may be. How Much Is Surface Tension Of Water.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension How Much Is Surface Tension Of Water Surface tension may be expressed, therefore, in units of energy per unit area (square metres). Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. It seems to defy the laws of physics, but a paper clip made of steel can indeed float on the water surface.. How Much Is Surface Tension Of Water.