Alkaline Battery Vs Acid Battery . Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. This battery is called an alkaline. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery.
from batteryguy.com
The second type is rechargeable and is called a secondary battery. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. Alkaline batteries use a basic electrolyte made of potassium hydroxide. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. This battery is called an alkaline. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights.
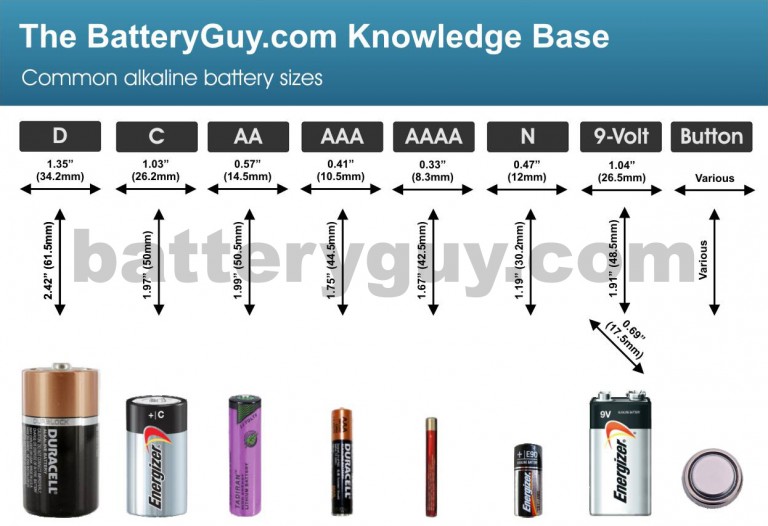
What are Alkaline Batteries? Knowledge Base
Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. The second type is rechargeable and is called a secondary battery. This battery is called an alkaline. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries use a basic electrolyte made of potassium hydroxide.
From ar.inspiredpencil.com
Alkaline Battery Diagram Alkaline Battery Vs Acid Battery Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls,. Alkaline Battery Vs Acid Battery.
From ioncoretechnology.com
What is the Difference Between Alkaline and Lithium Batteries? Alkaline Battery Vs Acid Battery Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. This battery is called an alkaline. Alkaline batteries are typically. Alkaline Battery Vs Acid Battery.
From www.artofit.org
Lithium battery vs regular alkaline battery which is better Artofit Alkaline Battery Vs Acid Battery Dry cells and (most) alkaline batteries are examples of primary batteries. This battery is called an alkaline. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are typically used in portable electronic devices and have. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Alkaline Battery Vs Acid Battery Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are a. Alkaline Battery Vs Acid Battery.
From discover.hubpages.com
Advantages of Alkaline Battery over Lead Acid Battery HubPages Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. Dry cells and (most) alkaline batteries are examples of primary. Alkaline Battery Vs Acid Battery.
From www.redwaypower.com
Rechargeable Lithium Batteries vs Alkaline Batteries Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. This battery is called an alkaline. The second type is rechargeable and is called a secondary battery. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. Alkaline. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Alkaline Battery Vs Acid Battery Alkaline batteries use a basic electrolyte made of potassium hydroxide. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. The second type is rechargeable and is called a secondary battery. Lead acid batteries are rechargeable, use lead plates. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. They are called alkaline batteries because they are made with an. Alkaline Battery Vs Acid Battery.
From energytheory.com
Alkaline Battery vs Lithium BatteryWhich is Better? Energy Theory Alkaline Battery Vs Acid Battery Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. Dry cells and (most) alkaline batteries are examples of primary batteries. This battery is called an alkaline. Alkaline batteries are typically used in portable electronic devices and have a higher energy density,. Alkaline Battery Vs Acid Battery.
From npplithium.com
Lithium vs Alkaline Batteries Comparison Analysis NPP POWER Alkaline Battery Vs Acid Battery Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. This battery is called an alkaline. The second type is rechargeable and is called a secondary battery. Alkaline batteries are typically used in portable electronic devices and. Alkaline Battery Vs Acid Battery.
From batteryguy.com
What are Alkaline Batteries? Knowledge Base Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. This battery is called an alkaline. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead. Alkaline Battery Vs Acid Battery.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Dry cells and (most) alkaline batteries are examples of primary batteries. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and. Alkaline Battery Vs Acid Battery.
From npplithium.com
Lithium vs Alkaline Batteries, Which is better? What's the Difference Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. This battery is called an alkaline. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
Lithium vs alkaline batteries where the differences and which is Alkaline Battery Vs Acid Battery Alkaline batteries use a basic electrolyte made of potassium hydroxide. Dry cells and (most) alkaline batteries are examples of primary batteries. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries are typically used in portable electronic devices and have a higher. Alkaline Battery Vs Acid Battery.
From www.youtube.com
Batteries Alkaline vs NiMH vs Lithiumion YouTube Alkaline Battery Vs Acid Battery They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. This battery is called an alkaline. The second type is rechargeable and is called a secondary battery. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries use a basic electrolyte. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Alkaline Battery Vs Acid Battery This battery is called an alkaline. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable,. Alkaline Battery Vs Acid Battery.
From differencesfinder.com
What is the Difference Between Lithium and Alkaline Batteries Alkaline Battery Vs Acid Battery Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. This battery is called an alkaline. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing. Alkaline Battery Vs Acid Battery.
From in.pinterest.com
Alkaline vs. Lithium Batteries What's The Difference (With Table Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries use a basic electrolyte made of potassium hydroxide. This battery. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
The difference between alkaline battery and lead acid battery TYCORUN Alkaline Battery Vs Acid Battery Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Dry cells and (most) alkaline batteries are examples of primary. Alkaline Battery Vs Acid Battery.
From makermax.ca
Experts in Battery Technology Makermax Inc. Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are typically used in. Alkaline Battery Vs Acid Battery.
From markets.financialcontent.com
Lithium vs Alkaline Batteries Ultimate Guide by Redway Battery BPAS Alkaline Battery Vs Acid Battery They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. This battery is called an alkaline. Alkaline batteries use a basic electrolyte. Alkaline Battery Vs Acid Battery.
From en.wikipedia.org
Alkaline battery Wikipedia Alkaline Battery Vs Acid Battery Alkaline batteries use a basic electrolyte made of potassium hydroxide. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. Alkaline batteries are a. Alkaline Battery Vs Acid Battery.
From www.askdifference.com
Lead Acid Battery vs. Alkaline Battery — What’s the Difference? Alkaline Battery Vs Acid Battery Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Alkaline batteries use a basic electrolyte made of potassium hydroxide.. Alkaline Battery Vs Acid Battery.
From electrical4u.com
Alkaline Batteries Electrical4u Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and power. They are called alkaline batteries because they are made with. Alkaline Battery Vs Acid Battery.
From www.slarbattery.com
The Ultimate Battery Showdown Lithium vs Alkaline Batteries SLAR Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and. Alkaline Battery Vs Acid Battery.
From survivalistprepper.net
The Best Batteries for Emergency Preparedness & Storage Survivalist Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. Dry cells and (most) alkaline batteries are examples of primary batteries. This battery is called an alkaline. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead acid batteries are rechargeable, use lead plates and sulfuric. Alkaline Battery Vs Acid Battery.
From www.arrow.com
The Best Battery for Your Build Alkaline Battery Vs Acid Battery They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Dry cells and (most) alkaline batteries are examples of primary batteries. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Alkaline. Alkaline Battery Vs Acid Battery.
From www.takomabattery.com
Comparison guide for carbon zinc battery vs alkaline TYCORUN ENERGY Alkaline Battery Vs Acid Battery Alkaline batteries use a basic electrolyte made of potassium hydroxide. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. This battery is called an alkaline. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use. Alkaline Battery Vs Acid Battery.
From differencebtw.com
Lead Acid Battery vs. Alkaline Battery Know the Difference Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. This battery is called an alkaline. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that. Alkaline Battery Vs Acid Battery.
From www.electricity-magnetism.org
Characteristics of Alkaline Batteries Cell voltage, Capacity & Self Alkaline Battery Vs Acid Battery Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Alkaline batteries are a type of primary battery that is commonly used. Alkaline Battery Vs Acid Battery.
From www.electricity-magnetism.org
Alkaline Battery Description & Application Electricity Alkaline Battery Vs Acid Battery This battery is called an alkaline. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. The second type is rechargeable and is called a secondary battery. Dry cells and (most) alkaline batteries are examples of primary batteries. Lead acid batteries are rechargeable, use. Alkaline Battery Vs Acid Battery.
From www.youtube.com
Top 5 Best Alkaline Batteries [Tested & Reviewed] YouTube Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. This battery is called an alkaline. The second type is rechargeable and is called a secondary battery. Alkaline batteries are typically used in portable electronic devices and have a higher energy density, allowing them to last longer. They. Alkaline Battery Vs Acid Battery.
From www.evssupply.com
Alkaline vs. Lithium Batteries EVS Supply Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. They are called alkaline batteries because they are made with an alkaline electrolyte solution, which is a type of chemical that has a high ph value. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc. Alkaline Battery Vs Acid Battery.
From nerdytechy.com
Lithium vs. Alkaline Batteries What's the Difference? NerdyTechy Alkaline Battery Vs Acid Battery The second type is rechargeable and is called a secondary battery. Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and. Alkaline Battery Vs Acid Battery.
From www.electricity-magnetism.org
Why are alkaline batteries (AAA or AA) made to be 1.5V while Alkaline Battery Vs Acid Battery Alkaline batteries are a type of primary battery that is commonly used in household items such as remote controls, toys, and flashlights. Dry cells and (most) alkaline batteries are examples of primary batteries. Lead acid batteries are rechargeable, use lead plates and sulfuric acid, and are often in vehicles, while alkaline batteries are disposable, use zinc and manganese dioxide, and. Alkaline Battery Vs Acid Battery.