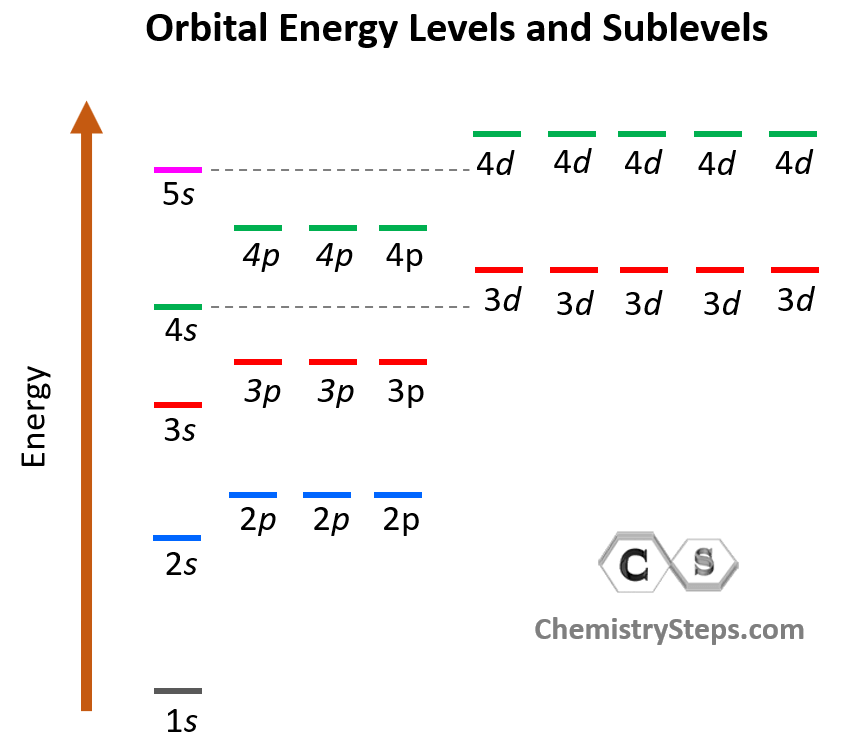
Energy Levels Spdf . This video explains s, p, d, and f orbitals, sublevels, and their shapes. The spdf notation (also called spectroscopic notation). 1s is lower energy than 2s which is lower energy than 3s; For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. These orbitals have different shapes (e.g. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. There are multiple orbitals within an atom. The number in front of the energy level indicates relative energy. Although the distributions of electrons in each orbital are. Two methods are used to represent this electron configuration. It discusses the 4 quantum. Because each orbital is different, they are assigned specific quantum numbers: Each has its own specific energy level and properties. The most common way to describe electron configurations is to write distributions in the spdf notation. Electron density distributions in space) and energies (e.g.
from general.chemistrysteps.com
Each has its own specific energy level and properties. For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. Although the distributions of electrons in each orbital are. Because each orbital is different, they are assigned specific quantum numbers: There are multiple orbitals within an atom. The most common way to describe electron configurations is to write distributions in the spdf notation. The spdf notation (also called spectroscopic notation). This video explains s, p, d, and f orbitals, sublevels, and their shapes. The number in front of the energy. 1s is lower energy than 2s which is lower energy than 3s;
s, p, d, f Atomic Orbitals Chemistry Steps
Energy Levels Spdf Because each orbital is different, they are assigned specific quantum numbers: It discusses the 4 quantum. For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. The number in front of the energy level indicates relative energy. Although the distributions of electrons in each orbital are. The spdf notation (also called spectroscopic notation). ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. There are multiple orbitals within an atom. Each has its own specific energy level and properties. 1s is lower energy than 2s which is lower energy than 3s; The number in front of the energy. Electron density distributions in space) and energies (e.g. Two methods are used to represent this electron configuration. This video explains s, p, d, and f orbitals, sublevels, and their shapes. These orbitals have different shapes (e.g. The most common way to describe electron configurations is to write distributions in the spdf notation.
From chemistryismyjam.com
The Atom Chemistry Is My Jam! Energy Levels Spdf This video explains s, p, d, and f orbitals, sublevels, and their shapes. Each has its own specific energy level and properties. Two methods are used to represent this electron configuration. Because each orbital is different, they are assigned specific quantum numbers: The number in front of the energy level indicates relative energy. The number in front of the energy.. Energy Levels Spdf.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Energy Levels Spdf The most common way to describe electron configurations is to write distributions in the spdf notation. Although the distributions of electrons in each orbital are. Because each orbital is different, they are assigned specific quantum numbers: This video explains s, p, d, and f orbitals, sublevels, and their shapes. ⚛ the highest energy level (valence shell) of a group 13. Energy Levels Spdf.
From www.youtube.com
Electron Configurations SPDF and Kernel Notation Notes YouTube Energy Levels Spdf Two methods are used to represent this electron configuration. There are multiple orbitals within an atom. Although the distributions of electrons in each orbital are. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. Each has its own specific energy level and. Energy Levels Spdf.
From wiringfixunripping.z21.web.core.windows.net
Valence Electrons Orbital Diagram Energy Levels Spdf There are multiple orbitals within an atom. The number in front of the energy level indicates relative energy. The number in front of the energy. 1s is lower energy than 2s which is lower energy than 3s; For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. ⚛ the highest energy level (valence shell). Energy Levels Spdf.
From www.vrogue.co
A Chart Of The Spdf Electron Orbitals Chemistry Educa vrogue.co Energy Levels Spdf There are multiple orbitals within an atom. Two methods are used to represent this electron configuration. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. The number in front of the energy. Electron density distributions in space) and energies (e.g. The spdf. Energy Levels Spdf.
From resolutionsforyou.com
Schematic energy level diagram Energy Levels Spdf There are multiple orbitals within an atom. Two methods are used to represent this electron configuration. The number in front of the energy level indicates relative energy. 1s is lower energy than 2s which is lower energy than 3s; For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. The spdf notation (also called. Energy Levels Spdf.
From slideplayer.com
Unit 4 Topic 1 SPDF. Principal Energy Levels Electrons occupy principal Energy Levels Spdf Although the distributions of electrons in each orbital are. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. The number in front of the energy level indicates relative energy. These orbitals have different shapes (e.g. The spdf notation (also called spectroscopic notation).. Energy Levels Spdf.
From slideplayer.com
Unit 4 Topic 1 SPDF. Principal Energy Levels Electrons occupy principal Energy Levels Spdf The spdf notation (also called spectroscopic notation). This video explains s, p, d, and f orbitals, sublevels, and their shapes. The most common way to describe electron configurations is to write distributions in the spdf notation. It discusses the 4 quantum. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s. Energy Levels Spdf.
From schematicfixpulpits.z21.web.core.windows.net
Electron Configuration Energy Level Diagram Energy Levels Spdf 1s is lower energy than 2s which is lower energy than 3s; The number in front of the energy level indicates relative energy. The spdf notation (also called spectroscopic notation). The most common way to describe electron configurations is to write distributions in the spdf notation. ⚛ the highest energy level (valence shell) of a group 13 element already has. Energy Levels Spdf.
From www.youtube.com
Electron configuration spdf notation Part 2 YouTube Energy Levels Spdf The most common way to describe electron configurations is to write distributions in the spdf notation. There are multiple orbitals within an atom. The number in front of the energy. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. Because each orbital. Energy Levels Spdf.
From animalia-life.club
Electron Energy Levels Chart Energy Levels Spdf It discusses the 4 quantum. This video explains s, p, d, and f orbitals, sublevels, and their shapes. Because each orbital is different, they are assigned specific quantum numbers: 1s is lower energy than 2s which is lower energy than 3s; Two methods are used to represent this electron configuration. For example, 1s is lower energy than 2s, which in. Energy Levels Spdf.
From thomas-hammack.blogspot.com
Spdf Orbitals Energy Levels Experts What Is The Correct Order Of Energy Levels Spdf The number in front of the energy. The spdf notation (also called spectroscopic notation). It discusses the 4 quantum. For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. Because each orbital is different, they are assigned specific quantum numbers: Electron density distributions in space) and energies (e.g. This video explains s, p, d,. Energy Levels Spdf.
From chemsite.lsrhs.net
Electron Configurations Energy Levels Spdf For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. Because each orbital is different, they are assigned specific quantum numbers: 1s is lower energy than 2s which is lower energy than 3s; Although the distributions of electrons in each orbital are. The number in front of the energy level indicates relative energy. These. Energy Levels Spdf.
From slideplayer.com
Atomic Structure. ppt download Energy Levels Spdf The number in front of the energy. Electron density distributions in space) and energies (e.g. Although the distributions of electrons in each orbital are. 1s is lower energy than 2s which is lower energy than 3s; Because each orbital is different, they are assigned specific quantum numbers: The spdf notation (also called spectroscopic notation). The number in front of the. Energy Levels Spdf.
From niam-carney.blogspot.com
Spdf Chart Niam Carney Energy Levels Spdf Although the distributions of electrons in each orbital are. The most common way to describe electron configurations is to write distributions in the spdf notation. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. The number in front of the energy level. Energy Levels Spdf.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Energy Levels Spdf The most common way to describe electron configurations is to write distributions in the spdf notation. Electron density distributions in space) and energies (e.g. The number in front of the energy level indicates relative energy. Each has its own specific energy level and properties. For example, 1s is lower energy than 2s, which in turn is lower energy than 2p.. Energy Levels Spdf.
From www.slideshare.net
Quantum mechanics Energy Levels Spdf The spdf notation (also called spectroscopic notation). It discusses the 4 quantum. 1s is lower energy than 2s which is lower energy than 3s; ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. These orbitals have different shapes (e.g. The number in. Energy Levels Spdf.
From pages.swcp.com
Parsing the spdf electron orbital model Energy Levels Spdf ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. The number in front of the energy level indicates relative energy. Although the distributions of electrons in each orbital are. This video explains s, p, d, and f orbitals, sublevels, and their shapes.. Energy Levels Spdf.
From utedzz.blogspot.com
Spdf Periodic Table Energy Levels Periodic Table Timeline Energy Levels Spdf The number in front of the energy level indicates relative energy. Although the distributions of electrons in each orbital are. Two methods are used to represent this electron configuration. Electron density distributions in space) and energies (e.g. The number in front of the energy. There are multiple orbitals within an atom. These orbitals have different shapes (e.g. This video explains. Energy Levels Spdf.
From www.thoughtco.com
Electronic Structure and the Aufbau Principle Energy Levels Spdf The spdf notation (also called spectroscopic notation). Electron density distributions in space) and energies (e.g. There are multiple orbitals within an atom. Each has its own specific energy level and properties. ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. This video. Energy Levels Spdf.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Energy Levels Spdf Each has its own specific energy level and properties. This video explains s, p, d, and f orbitals, sublevels, and their shapes. Two methods are used to represent this electron configuration. The most common way to describe electron configurations is to write distributions in the spdf notation. These orbitals have different shapes (e.g. The spdf notation (also called spectroscopic notation).. Energy Levels Spdf.
From www.youtube.com
Chemistry Orbitals and Energy Levels, spdf YouTube Energy Levels Spdf These orbitals have different shapes (e.g. Electron density distributions in space) and energies (e.g. The spdf notation (also called spectroscopic notation). Because each orbital is different, they are assigned specific quantum numbers: The number in front of the energy. 1s is lower energy than 2s which is lower energy than 3s; The number in front of the energy level indicates. Energy Levels Spdf.
From ar.inspiredpencil.com
Electron Orbitals S P D F Energy Levels Spdf For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. Because each orbital is different, they are assigned specific quantum numbers: Electron density distributions in space) and energies (e.g. Two methods are used to represent this electron configuration. These orbitals have different shapes (e.g. The number in front of the energy level indicates relative. Energy Levels Spdf.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps Energy Levels Spdf The number in front of the energy. The number in front of the energy level indicates relative energy. Because each orbital is different, they are assigned specific quantum numbers: ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. Although the distributions of. Energy Levels Spdf.
From www.vrogue.co
A Chart Of The Spdf Electron Orbitals Chemistry Educa vrogue.co Energy Levels Spdf 1s is lower energy than 2s which is lower energy than 3s; This video explains s, p, d, and f orbitals, sublevels, and their shapes. The number in front of the energy level indicates relative energy. The most common way to describe electron configurations is to write distributions in the spdf notation. The spdf notation (also called spectroscopic notation). Although. Energy Levels Spdf.
From ibalchemy.com
3.1 Periodic table IB Alchemy Energy Levels Spdf The number in front of the energy. 1s is lower energy than 2s which is lower energy than 3s; ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. Two methods are used to represent this electron configuration. These orbitals have different shapes. Energy Levels Spdf.
From utedzz.blogspot.com
Spdf Periodic Table Energy Levels Periodic Table Timeline Energy Levels Spdf The number in front of the energy. It discusses the 4 quantum. Each has its own specific energy level and properties. The most common way to describe electron configurations is to write distributions in the spdf notation. These orbitals have different shapes (e.g. The number in front of the energy level indicates relative energy. There are multiple orbitals within an. Energy Levels Spdf.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table Energy Levels Spdf Each has its own specific energy level and properties. These orbitals have different shapes (e.g. The spdf notation (also called spectroscopic notation). 1s is lower energy than 2s which is lower energy than 3s; Because each orbital is different, they are assigned specific quantum numbers: Although the distributions of electrons in each orbital are. This video explains s, p, d,. Energy Levels Spdf.
From chemwiki.ucdavis.edu
Electron Configuration of Transition Metals Chemwiki Energy Levels Spdf Electron density distributions in space) and energies (e.g. Each has its own specific energy level and properties. For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. 1s is lower energy than 2s which is lower energy than 3s; The number in front of the energy level indicates relative energy. These orbitals have different. Energy Levels Spdf.
From chem.libretexts.org
2.4 Electron Configurations Chemistry LibreTexts Energy Levels Spdf The number in front of the energy level indicates relative energy. Because each orbital is different, they are assigned specific quantum numbers: Each has its own specific energy level and properties. This video explains s, p, d, and f orbitals, sublevels, and their shapes. 1s is lower energy than 2s which is lower energy than 3s; Electron density distributions in. Energy Levels Spdf.
From pages.swcp.com
Parsing the spdf electron orbital model Energy Levels Spdf Although the distributions of electrons in each orbital are. The spdf notation (also called spectroscopic notation). ⚛ the highest energy level (valence shell) of a group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p. These orbitals have different shapes (e.g. The number in front of the energy. The most common way. Energy Levels Spdf.
From socratic.org
Can someone compare s, p, d, and f orbitals in terms of size, shape Energy Levels Spdf 1s is lower energy than 2s which is lower energy than 3s; The number in front of the energy level indicates relative energy. There are multiple orbitals within an atom. The most common way to describe electron configurations is to write distributions in the spdf notation. ⚛ the highest energy level (valence shell) of a group 13 element already has. Energy Levels Spdf.
From www.youtube.com
SPDF Sublevels YouTube Energy Levels Spdf Although the distributions of electrons in each orbital are. 1s is lower energy than 2s which is lower energy than 3s; For example, 1s is lower energy than 2s, which in turn is lower energy than 2p. The spdf notation (also called spectroscopic notation). Two methods are used to represent this electron configuration. Each has its own specific energy level. Energy Levels Spdf.
From utedzz.blogspot.com
Spdf Periodic Table Energy Levels Periodic Table Timeline Energy Levels Spdf There are multiple orbitals within an atom. 1s is lower energy than 2s which is lower energy than 3s; These orbitals have different shapes (e.g. The most common way to describe electron configurations is to write distributions in the spdf notation. This video explains s, p, d, and f orbitals, sublevels, and their shapes. The number in front of the. Energy Levels Spdf.
From chem.libretexts.org
7.2 Atomic Subshell Energies and Electron Assignments Chemistry Energy Levels Spdf This video explains s, p, d, and f orbitals, sublevels, and their shapes. 1s is lower energy than 2s which is lower energy than 3s; Because each orbital is different, they are assigned specific quantum numbers: These orbitals have different shapes (e.g. Electron density distributions in space) and energies (e.g. The most common way to describe electron configurations is to. Energy Levels Spdf.