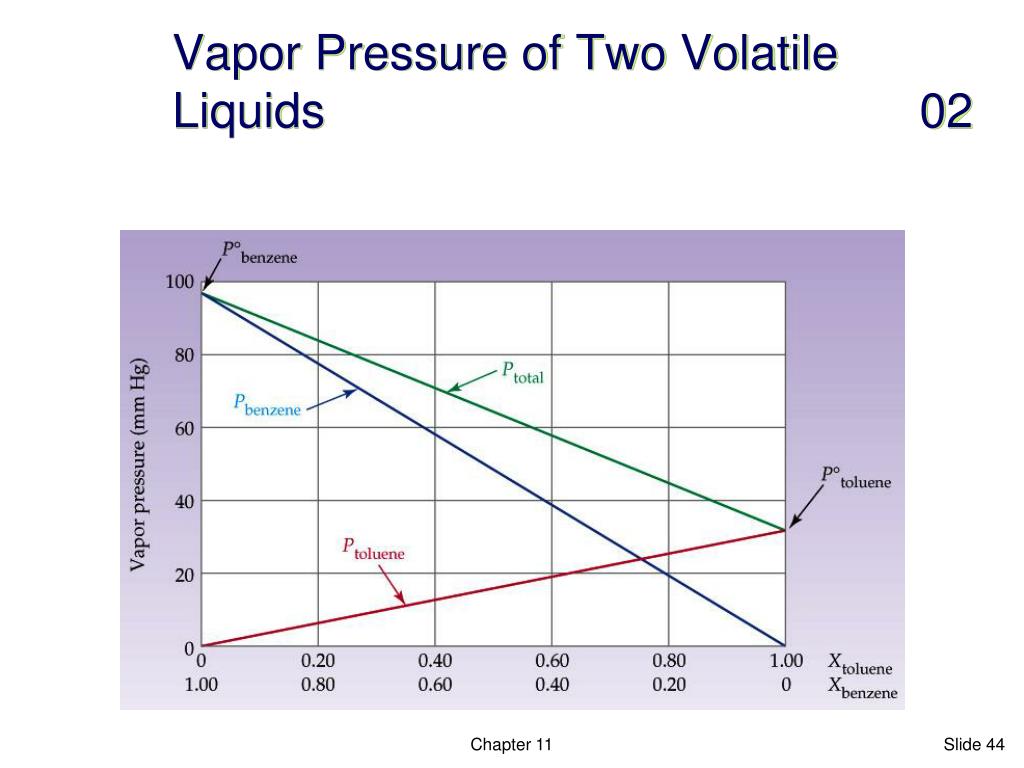
Vapor Pressure Volatility . Volatility is directly related to a substance's vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). So a highly volatile liquid would show a great tendency to evaporate. Volatility is essentially the tendency of a liquid to evaporate. Volatility is not measured directly, but a. In chemistry and physics, volatility is the tendency of a substance to vaporize. A measure of volatility is the vapor pressure.
from www.slideserve.com
Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is not measured directly, but a. A measure of volatility is the vapor pressure. Volatility is directly related to a substance's vapor. So a highly volatile liquid would show a great tendency to evaporate. In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is essentially the tendency of a liquid to evaporate.
PPT Solutions and Their Properties PowerPoint Presentation, free
Vapor Pressure Volatility Volatility is not measured directly, but a. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is not measured directly, but a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. A measure of volatility is the vapor pressure. Volatility is essentially the tendency of a liquid to evaporate. So a highly volatile liquid would show a great tendency to evaporate. Volatility is directly related to a substance's vapor.
From www.slideserve.com
PPT Vapor Pressure and Changes of State PowerPoint Presentation, free Vapor Pressure Volatility Volatility is directly related to a substance's vapor. Volatility is not measured directly, but a. A measure of volatility is the vapor pressure. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. In chemistry. Vapor Pressure Volatility.
From www.slideserve.com
PPT Solutions and Their Properties PowerPoint Presentation, free Vapor Pressure Volatility A measure of volatility is the vapor pressure. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is not measured directly, but a. So a highly volatile liquid would show a great tendency to evaporate.. Vapor Pressure Volatility.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Vapor Pressure Volatility Volatility is not measured directly, but a. In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor. A measure of volatility is the vapor pressure. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is essentially the. Vapor Pressure Volatility.
From www.slideserve.com
PPT Intermolecular Forces Phases of Matter & Colligative Properties Vapor Pressure Volatility Volatility is directly related to a substance's vapor. A measure of volatility is the vapor pressure. In chemistry and physics, volatility is the tendency of a substance to vaporize. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is essentially the tendency of a liquid to evaporate. So a highly volatile liquid would. Vapor Pressure Volatility.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 Vapor Pressure Volatility In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor. A measure of volatility is the vapor pressure. Volatility is not measured directly, but a. Volatility is essentially the tendency of a liquid to evaporate. So a highly volatile liquid would show a great tendency to evaporate. Generally a. Vapor Pressure Volatility.
From www.semanticscholar.org
Figure 1 from Quantum chemical calculation of the vapor pressure of Vapor Pressure Volatility A measure of volatility is the vapor pressure. In chemistry and physics, volatility is the tendency of a substance to vaporize. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is not measured directly, but a. So a highly volatile liquid would show a great tendency to evaporate. Volatility is a measure of. Vapor Pressure Volatility.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID7080797 Vapor Pressure Volatility So a highly volatile liquid would show a great tendency to evaporate. A measure of volatility is the vapor pressure. Volatility is not measured directly, but a. Volatility is directly related to a substance's vapor. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is essentially the tendency. Vapor Pressure Volatility.
From www.youtube.com
Vapor Pressure and Volatility YouTube Vapor Pressure Volatility A measure of volatility is the vapor pressure. So a highly volatile liquid would show a great tendency to evaporate. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is essentially the tendency. Vapor Pressure Volatility.
From www.slideserve.com
PPT Solution Properties PowerPoint Presentation, free download ID Vapor Pressure Volatility Volatility is directly related to a substance's vapor. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). So a highly volatile liquid would show a great tendency to evaporate. A measure of volatility is the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as. Vapor Pressure Volatility.
From www.youtube.com
AP Chemistry © U08.03 Volatility and Vapor Pressure YouTube Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is not measured directly, but a. So a highly volatile liquid would show a great tendency to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is directly related to a. Vapor Pressure Volatility.
From www.researchgate.net
(Color) Vapor pressure values calculated for the most volatile reaction Vapor Pressure Volatility Volatility is essentially the tendency of a liquid to evaporate. A measure of volatility is the vapor pressure. Volatility is directly related to a substance's vapor. Volatility is not measured directly, but a. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Generally a substance's vapor pressure increases as. Vapor Pressure Volatility.
From www.slideserve.com
PPT Solutions and Their Properties PowerPoint Presentation, free Vapor Pressure Volatility A measure of volatility is the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is directly related to a substance's vapor. In chemistry and physics, volatility is the tendency of a substance to vaporize. So a highly volatile liquid would show a great tendency to evaporate. Volatility is not measured. Vapor Pressure Volatility.
From www.youtube.com
CHEM 201 Finding mole fraction from vapor pressure of a mixture with Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is not measured directly, but a. So a highly volatile liquid would show a great tendency to evaporate. In chemistry and physics, volatility is. Vapor Pressure Volatility.
From www.doubtnut.com
The vapour pressure curves of the same solute in the same solvent are Vapor Pressure Volatility A measure of volatility is the vapor pressure. So a highly volatile liquid would show a great tendency to evaporate. Volatility is not measured directly, but a. In chemistry and physics, volatility is the tendency of a substance to vaporize. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is a measure of. Vapor Pressure Volatility.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is essentially the tendency of a liquid to evaporate. A measure of volatility is the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is directly related to a substance's. Vapor Pressure Volatility.
From www.mdpi.com
ChemEngineering Free FullText Vapor Pressure Mapping of Ionic Vapor Pressure Volatility Volatility is directly related to a substance's vapor. So a highly volatile liquid would show a great tendency to evaporate. In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is essentially the tendency of a. Vapor Pressure Volatility.
From studylib.net
Vapor Pressure and Volatile Solutes Ideal Solutions Vapor Pressure Volatility Volatility is directly related to a substance's vapor. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is not measured directly, but a. Volatility is essentially the tendency of a liquid to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Vapor Pressure Volatility.
From www.slideserve.com
PPT Topic 12 Solutions PowerPoint Presentation, free download ID Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). So a highly volatile liquid would show a great tendency to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is directly related to a substance's vapor. A measure of volatility is. Vapor Pressure Volatility.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation Vapor Pressure Volatility Volatility is directly related to a substance's vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. So a highly volatile liquid would show a great tendency to evaporate. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is not measured directly,. Vapor Pressure Volatility.
From www.sliderbase.com
Vapor Pressure of Solutions Presentation Chemistry Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. A measure of volatility is the vapor pressure. So a highly volatile liquid would show a great tendency to evaporate. Volatility is directly related to. Vapor Pressure Volatility.
From digital.library.unt.edu
VaporPressure Chart for Volatile Hydrocarbons Page 3 UNT Digital Vapor Pressure Volatility Volatility is not measured directly, but a. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is essentially the tendency of a liquid to evaporate. A measure of volatility is the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Vapor Pressure Volatility.
From www.youtube.com
UC Merced LAIR CHEM10, Sec01 Chapter 11 Vapor Pressure Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is not measured directly, but a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. So a highly volatile liquid would show a great tendency to evaporate. Volatility is essentially the tendency of. Vapor Pressure Volatility.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3115730 Vapor Pressure Volatility Volatility is not measured directly, but a. A measure of volatility is the vapor pressure. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor. Generally a substance's vapor. Vapor Pressure Volatility.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5347387 Vapor Pressure Volatility Volatility is essentially the tendency of a liquid to evaporate. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). So a highly volatile liquid would show a great tendency to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is not. Vapor Pressure Volatility.
From www.slideserve.com
PPT Vapor Pressure and Changes of State PowerPoint Presentation, free Vapor Pressure Volatility Volatility is essentially the tendency of a liquid to evaporate. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor. A measure of volatility is the vapor pressure. Generally. Vapor Pressure Volatility.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Vapor Pressure Volatility A measure of volatility is the vapor pressure. So a highly volatile liquid would show a great tendency to evaporate. Volatility is directly related to a substance's vapor. Volatility is not measured directly, but a. In chemistry and physics, volatility is the tendency of a substance to vaporize. Generally a substance's vapor pressure increases as temperature increases and decreases as. Vapor Pressure Volatility.
From www.youtube.com
IMF/phases Volatility and vapor pressure YouTube Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). A measure of volatility is the vapor pressure. Volatility is essentially the tendency of a liquid to evaporate. Volatility is not measured directly, but a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Vapor Pressure Volatility.
From www.slideserve.com
PPT Distillation PowerPoint Presentation, free download ID6664017 Vapor Pressure Volatility Volatility is essentially the tendency of a liquid to evaporate. Volatility is directly related to a substance's vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In chemistry and physics, volatility is the. Vapor Pressure Volatility.
From www.slideserve.com
PPT Emergency Response to Terrorism TC Hazardous Materials Vapor Pressure Volatility Volatility is not measured directly, but a. A measure of volatility is the vapor pressure. So a highly volatile liquid would show a great tendency to evaporate. Volatility is essentially the tendency of a liquid to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. In chemistry and physics, volatility is the tendency. Vapor Pressure Volatility.
From www.mdpi.com
ChemEngineering Free FullText Vapor Pressure Mapping of Ionic Vapor Pressure Volatility A measure of volatility is the vapor pressure. Volatility is directly related to a substance's vapor. In chemistry and physics, volatility is the tendency of a substance to vaporize. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is a measure of how easily a material transitions into the gas phase via evaporation. Vapor Pressure Volatility.
From www.researchgate.net
b. Temperature dependence of the vapour pressure of the more volatile Vapor Pressure Volatility A measure of volatility is the vapor pressure. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is directly related to a substance's vapor. So a highly volatile liquid would show a great tendency to evaporate. Volatility is not measured directly, but a. Volatility is essentially the tendency. Vapor Pressure Volatility.
From courses.lumenlearning.com
Phase Changes Boundless Chemistry Vapor Pressure Volatility Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is directly related to a substance's vapor. Volatility is not measured directly, but a. Volatility is essentially the tendency of a liquid to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Vapor Pressure Volatility.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Vapor Pressure Volatility So a highly volatile liquid would show a great tendency to evaporate. A measure of volatility is the vapor pressure. Volatility is not measured directly, but a. Volatility is essentially the tendency of a liquid to evaporate. Volatility is directly related to a substance's vapor. In chemistry and physics, volatility is the tendency of a substance to vaporize. Generally a. Vapor Pressure Volatility.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5347387 Vapor Pressure Volatility Volatility is directly related to a substance's vapor. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). A measure of volatility is the vapor pressure. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Volatility is essentially the tendency of a liquid to. Vapor Pressure Volatility.
From www.numerade.com
SOLVEDThis portion of a phase diagram shows the vaporpressure curves Vapor Pressure Volatility Volatility is not measured directly, but a. Volatility is essentially the tendency of a liquid to evaporate. A measure of volatility is the vapor pressure. Volatility is directly related to a substance's vapor. So a highly volatile liquid would show a great tendency to evaporate. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Vapor Pressure Volatility.