Titration With Edta . ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Ph for edta titration of fe 3+ is slightly higher than 1.00. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. edta complexometric titration. complex titration with edta.
from www.numerade.com
ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Ph for edta titration of fe 3+ is slightly higher than 1.00. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. complex titration with edta. edta complexometric titration. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations.
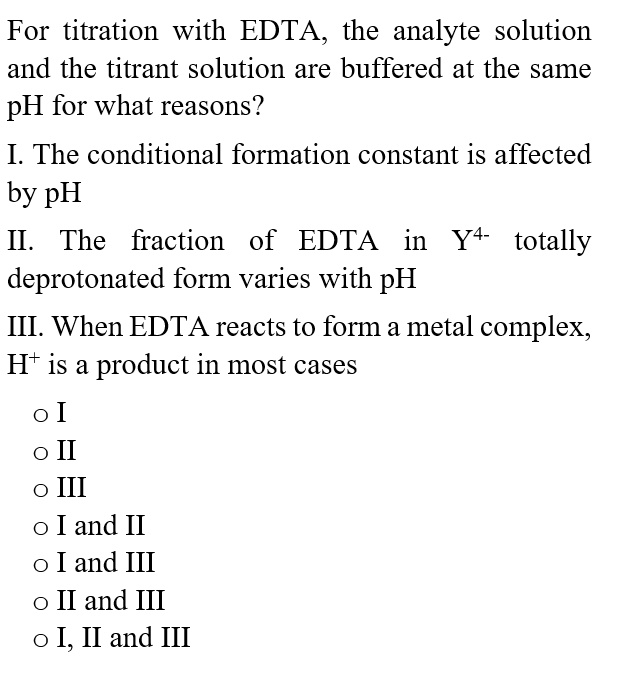
SOLVED For titration with EDTA, the analyte solution and the titrant
Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Ph for edta titration of fe 3+ is slightly higher than 1.00. edta complexometric titration. complex titration with edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases.
From www.youtube.com
EDTA Titrimetric Method (Experiment) Measurement of Total hardness, Ca Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. edta complexometric titration. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. It contains four carboxyl groups and two amine groups that function as electron. Titration With Edta.
From www.youtube.com
Calcium and Magnesium ion concentration determination with EDTA Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Ph for edta titration of fe 3+ is slightly higher than 1.00. ni 2+ can be analyzed. Titration With Edta.
From www.youtube.com
CaTitration with EDTA YouTube Titration With Edta edta complexometric titration. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. It contains four carboxyl groups. Titration With Edta.
From www.researchgate.net
The EDTA titration test of CSM (a) color change, and (b) EDTA Titration With Edta ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Ph for edta titration of fe 3+ is slightly higher than 1.00. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. edta complexometric titration. . Titration With Edta.
From www.vrogue.co
Modelling An Edta Titration Curve Youtube vrogue.co Titration With Edta ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Ph for edta titration of fe 3+ is slightly higher than 1.00. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. edta complexometric titration. It. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID1079499 Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Ph for edta titration of fe 3+ is slightly higher than 1.00. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5. Titration With Edta.
From thechemistrynotes.com
EDTA Titration, Types, Advantages, Disadvantages Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Ph for edta titration of fe 3+ is slightly higher than 1.00. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Ph for edta titration of fe 3+ is slightly higher than 1.00. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. edta complexometric titration. complex titration with edta. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Edta,. Titration With Edta.
From www.studocu.com
EDTA titration lab Lecture notes 7 Determination of Ca2+ and Mg2 Titration With Edta Ph for edta titration of fe 3+ is slightly higher than 1.00. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. edta complexometric titration. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. ni 2+ can be. Titration With Edta.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Titration With Edta Ph for edta titration of fe 3+ is slightly higher than 1.00. complex titration with edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. ni 2+ can be analyzed by back titration, using standard. Titration With Edta.
From www.youtube.com
Chemical experiment Complex titration with EDTA YouTube Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. complex titration with edta. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Ph for edta titration. Titration With Edta.
From www.slideshare.net
Complexometric Titration, EDTA Titration Titration With Edta even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Ph. Titration With Edta.
From www.numerade.com
SOLVED For titration with EDTA, the analyte solution and the titrant Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. edta complexometric titration. Ph for edta titration of fe. Titration With Edta.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Titration With Edta edta complexometric titration. Ph for edta titration of fe 3+ is slightly higher than 1.00. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. complex titration with edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration With Edta edta complexometric titration. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Ph for edta titration of fe 3+ is slightly higher than 1.00. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. ni 2+ can be analyzed by back titration, using standard zn. Titration With Edta.
From slideplayer.com
Chapter 11 EDTA Titrations ppt download Titration With Edta Ph for edta titration of fe 3+ is slightly higher than 1.00. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. complex titration with edta. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. edta complexometric titration. It contains four carboxyl groups and two. Titration With Edta.
From studylib.net
Determination of Calcium by Titration with EDTA Titration With Edta ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Ph for edta titration of fe 3+ is slightly higher than 1.00. edta complexometric titration. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. complex titration with edta. Edta, known as ethylenediaminetetraacetic acid, is the preferred. Titration With Edta.
From www.numerade.com
SOLVED Aluminum is determined by titration with EDTA A 1.00 g sample Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. complex titration with edta. edta complexometric titration. ni 2+ can be analyzed by back titration,. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Ph for edta titration of fe 3+ is slightly higher than 1.00. complex titration with edta. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Edta, known as ethylenediaminetetraacetic. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration With Edta ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. . Titration With Edta.
From www.youtube.com
Lab 5 Copper Titration with EDTA YouTube Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. complex titration with edta. edta complexometric titration. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. even if a suitable indicator does not exist, it is often possible to complete an edta titration by. Titration With Edta.
From www.vrogue.co
Modelling An Edta Titration Curve Youtube vrogue.co Titration With Edta Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Ph for edta titration of fe 3+ is slightly higher than 1.00. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. complex titration with edta. edta complexometric titration. Edta, known as ethylenediaminetetraacetic acid, is the preferred. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Titration With Edta Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Ph for edta titration of fe 3+ is slightly higher than 1.00. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation ID234018 Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. edta complexometric titration. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. Ph for edta titration of fe 3+ is slightly higher than 1.00. complex titration with edta. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5. Titration With Edta.
From thechemistrynotes.com
EDTA Titration, Types, Advantages, Disadvantages Titration With Edta edta complexometric titration. Ph for edta titration of fe 3+ is slightly higher than 1.00. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. complex. Titration With Edta.
From slidetodoc.com
Chapter 12 EDTA Titrations Overview 12 1 MetalChelate Titration With Edta edta complexometric titration. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Ph for edta titration of fe 3+ is slightly higher than 1.00. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. complex titration with edta. even if a suitable indicator does not. Titration With Edta.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Titration With Edta even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. ni 2+ can be analyzed by back titration,. Titration With Edta.
From www.youtube.com
Titration Zn with EDTA, Xylenolorange indicator YouTube Titration With Edta It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. edta complexometric titration. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Ph for edta titration of fe 3+ is slightly higher than 1.00. complex. Titration With Edta.
From www.youtube.com
Modelling an EDTA Titration Curve YouTube Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. edta complexometric titration. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. It contains four carboxyl groups and two amine groups that function as electron. Titration With Edta.
From studylib.net
Experiment 6 EDTA Titration of the Hardness of Water Titration With Edta Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID89337 Titration With Edta Ph for edta titration of fe 3+ is slightly higher than 1.00. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. complex titration with edta. edta complexometric titration. It contains four carboxyl groups and two. Titration With Edta.
From studylib.net
EDTA Titration with Mg2+ Titration With Edta complex titration with edta. It contains four carboxyl groups and two amine groups that function as electron pair donors, acting as lewis bases. Ph for edta titration of fe 3+ is slightly higher than 1.00. edta complexometric titration. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and. Titration With Edta.
From www.slideshare.net
complexometric titration, general chemistry assignment Titration With Edta edta complexometric titration. ni 2+ can be analyzed by back titration, using standard zn 2+ at ph 5.5 w/ xylenol orange indicator. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. complex titration with. Titration With Edta.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID89337 Titration With Edta edta complexometric titration. even if a suitable indicator does not exist, it is often possible to complete an edta titration by introducing. Edta, known as ethylenediaminetetraacetic acid, is the preferred choice in these titrations. Edta, ethylenediaminetetraacetic acid, has four carboxyl groups and two amine groups. It contains four carboxyl groups and two amine groups that function as electron. Titration With Edta.