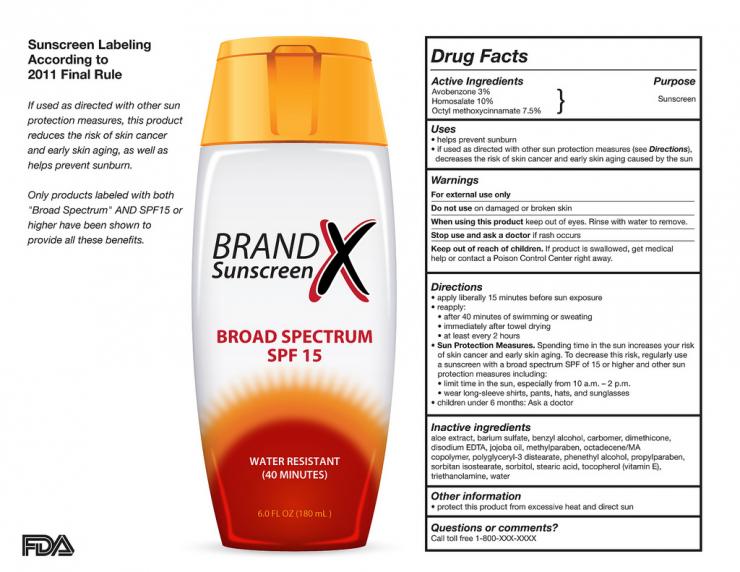
Fda Sunscreen Drug Facts Label . The labeling of the product contains the established. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation).
from www.medicalnewstoday.com
The labeling of the product contains the established. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. 352.52 labeling of sunscreen drug products.
Sun protection factor (SPF) What is the best sunscreen?
Fda Sunscreen Drug Facts Label The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established.
From www.medscape.org
Current Sun Protection A Clinical Update Fda Sunscreen Drug Facts Label 352.52 labeling of sunscreen drug products. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as. Fda Sunscreen Drug Facts Label.
From www.cbsnews.com
Sunscreen label rules issued by FDA aim to end confusion CBS News Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The fd&c act.(h) labeling of products containing a combination of sunscreen and. Fda Sunscreen Drug Facts Label.
From ndclist.com
NDC 31190700 Sunscreen Spf 30 Images Packaging, Labeling & Appearance Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. 352.52 labeling of sunscreen drug products. The labeling of the product contains the established. Fda Sunscreen Drug Facts Label.
From ndclist.com
Product Images Sunscreen Spf 30 Photos Packaging, Labels & Appearance Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an. Fda Sunscreen Drug Facts Label.
From www.enkoproducts.com
FDA Cosmetic Labeling Requirements and Label Printing Guide Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug Facts Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of. Fda Sunscreen Drug Facts Label.
From www.medicalnewstoday.com
Sun protection factor (SPF) What is the best sunscreen? Fda Sunscreen Drug Facts Label The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The labeling of the product contains the established. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999. Fda Sunscreen Drug Facts Label.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an. Fda Sunscreen Drug Facts Label.
From www.prweb.com
6 Days Until FDA Sunscreen Labeling Rule Compliance Deadline; Are You Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established name of the drug, if any, and identifies the product as. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug Facts Fda Sunscreen Drug Facts Label 352.52 labeling of sunscreen drug products. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The deemed final order for sunscreens includes certain. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
GoodSense SPF 30 Sunscreen Fda Sunscreen Drug Facts Label The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” A labeling designation for sunscreen drug products to aid. Fda Sunscreen Drug Facts Label.
From otclabels.com
Sunscreen SPF 50 Details from the FDA, via Fda Sunscreen Drug Facts Label The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” A labeling designation for sunscreen drug products to aid in selecting the type of product. Fda Sunscreen Drug Facts Label.
From otclabels.com
Surya Natural Mineral Sunscreen Details from the FDA, via Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. 352.52 labeling of. Fda Sunscreen Drug Facts Label.
From www.northshore.org
Sunscreen Facts Decoding Your Sunscreen Label NorthShore Fda Sunscreen Drug Facts Label The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's. Fda Sunscreen Drug Facts Label.
From otclabels.com
Everyday Sunscreen SPF 50 Details from the FDA, via Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. 352.52 labeling of sunscreen drug products. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established name of the drug, if. Fda Sunscreen Drug Facts Label.
From stylecaster.com
New Sunscreen FDA Regulations Get The Facts StyleCaster Fda Sunscreen Drug Facts Label 352.52 labeling of sunscreen drug products. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established. The labeling of the product. Fda Sunscreen Drug Facts Label.
From otclabels.com
SK1N NATURAL SUNSCREEN SPF 40 Details from the FDA, via Fda Sunscreen Drug Facts Label The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. 352.52 labeling of sunscreen. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug facts for sunscreen SPF 30 Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The deemed final. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug Facts Fda Sunscreen Drug Facts Label The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The deemed final order for sunscreens includes certain requirements. Fda Sunscreen Drug Facts Label.
From otclabels.com
Samhealthyskin B.B. SUNSCREEN BROAD SPECTRUM SPF 40 Details from the Fda Sunscreen Drug Facts Label The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product. Fda Sunscreen Drug Facts Label.
From www.cbsnews.com
Sunscreen label rules issued by FDA aim to end confusion CBS News Fda Sunscreen Drug Facts Label The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. A labeling designation for sunscreen drug products to aid. Fda Sunscreen Drug Facts Label.
From www.jwatch.org
Do People Understand the New FDA Sunscreen Labels? Fda Sunscreen Drug Facts Label The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph. Fda Sunscreen Drug Facts Label.
From otclabels.com
SUNNYLIFE SPF 50 SUNSCREEN COCONUT SCENTED Details from the FDA, via Fda Sunscreen Drug Facts Label 352.52 labeling of sunscreen drug products. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established name of the drug, if. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
SKIN STRONG SKIN EQUIPMENT SPF 30 SUNSCREEN LOTION DRUG FACTS LABEL Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the. Fda Sunscreen Drug Facts Label.
From otclabels.com
Equate SPF 50 Ultra Sunscreen Continuous Details from the FDA, via Fda Sunscreen Drug Facts Label The labeling of the product contains the established. 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The deemed final order for sunscreens includes certain requirements. Fda Sunscreen Drug Facts Label.
From otclabels.com
Baby Sunscreen SPF 50 Details from the FDA, via Fda Sunscreen Drug Facts Label 352.52 labeling of sunscreen drug products. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of the product contains the established. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The deemed final order for sunscreens includes certain. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug Facts Fda Sunscreen Drug Facts Label The labeling of the product contains the established. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established. Fda Sunscreen Drug Facts Label.
From otclabels.com
Elixir FINE Sunscreen Broad Spectrum SPF 15 Details from the FDA, via... Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). 352.52 labeling of sunscreen drug products. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The deemed final order for sunscreens includes certain requirements about active. Fda Sunscreen Drug Facts Label.
From www.scribd.com
Fda Fact SheetFda Proposed RuleSunscreen Drug Products PDF Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” A labeling designation for. Fda Sunscreen Drug Facts Label.
From www.livescience.com
Infographic What to Look for on New Sunscreen Labels Live Science Fda Sunscreen Drug Facts Label The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The labeling of. Fda Sunscreen Drug Facts Label.
From www.medscape.org
Current Sun Protection A Clinical Update Fda Sunscreen Drug Facts Label 352.52 labeling of sunscreen drug products. The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established name of the drug, if any, and identifies the product as. Fda Sunscreen Drug Facts Label.
From otclabels.com
7Eleven Broad Spectrum SPF Details from the FDA, via Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. The labeling of the product contains the established name of the drug, if any, and identifies the product as. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug Facts Fda Sunscreen Drug Facts Label The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The labeling of the product contains the established. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999 final monograph regulation for. A labeling designation for sunscreen drug products to aid in selecting the. Fda Sunscreen Drug Facts Label.
From otclabels.com
Equate Sunscreen SPF 50 BABY Broad Spectrum Details from the FDA, via... Fda Sunscreen Drug Facts Label The labeling of the product contains the established. The labeling of the product contains the established name of the drug, if any, and identifies the product as a “sunscreen.” The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. The deemed final order for sunscreens includes certain requirements about active ingredients from the 1999. Fda Sunscreen Drug Facts Label.
From dailymed.nlm.nih.gov
Drug Facts Fda Sunscreen Drug Facts Label A labeling designation for sunscreen drug products to aid in selecting the type of product best suited to an individual's complexion (pigmentation). The fd&c act.(h) labeling of products containing a combination of sunscreen and skin protectant active ingredients. 352.52 labeling of sunscreen drug products. The labeling of the product contains the established name of the drug, if any, and identifies. Fda Sunscreen Drug Facts Label.