Rate Constant K Of Crystal Violet . Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. What is the differential rate law for the hydroxylation of crystal violet. Hydroxylation of crystal violet is: One of these graphs will give a straight line; The chemical rate law consider the. The rate law for this reaction is in the form: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,.
from www.chegg.com
Hydroxylation of crystal violet is: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. The chemical rate law consider the. The rate law for this reaction is in the form: One of these graphs will give a straight line; The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. What is the differential rate law for the hydroxylation of crystal violet. Generally, the rate of the reaction depends on the concentration of one or more of the reactants.
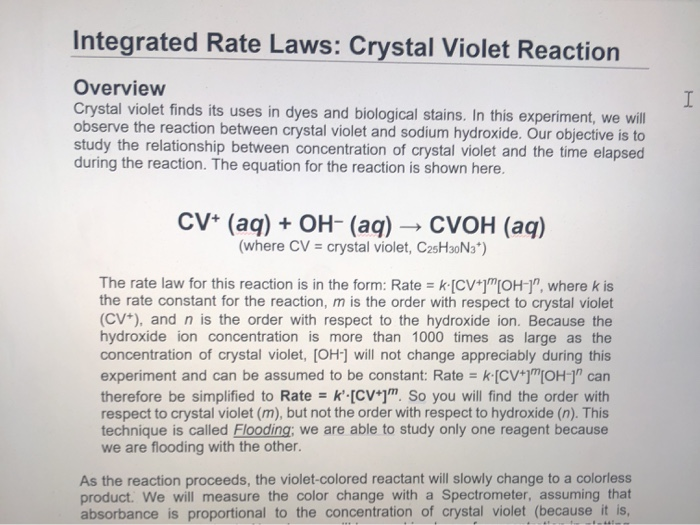
Integrated Rate Laws Crystal Violet Reaction I
Rate Constant K Of Crystal Violet The rate law for this reaction is in the form: What is the differential rate law for the hydroxylation of crystal violet. The chemical rate law consider the. The rate law for this reaction is in the form: Hydroxylation of crystal violet is: One of these graphs will give a straight line; The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,.
From es.scribd.com
of the reaction between crystal violet and hydroxide ions Rate Constant K Of Crystal Violet The rate law for this reaction is in the form: One of these graphs will give a straight line; The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Thus, the rate of the hypothetical reaction may. The chemical rate law consider the.. Rate Constant K Of Crystal Violet.
From www.chegg.com
What is the rate constant k, for this reaction, in Rate Constant K Of Crystal Violet The chemical rate law consider the. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. One of these graphs will give a straight line; Hydroxylation of crystal violet is: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and. Rate Constant K Of Crystal Violet.
From www.chegg.com
rate law determination of the crystal violet reaction Rate Constant K Of Crystal Violet Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The rate law for this reaction is in the form: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. Hydroxylation of crystal violet is: The chemical rate law consider. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved Name Partner DETERMINATION OF THE RATE LAW FOR Rate Constant K Of Crystal Violet Thus, the rate of the hypothetical reaction may. The rate law for this reaction is in the form: The chemical rate law consider the. What is the differential rate law for the hydroxylation of crystal violet. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved a student mixes crystal violet and sodium hydroxide Rate Constant K Of Crystal Violet Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The chemical rate law consider the. Hydroxylation of crystal violet is: The rate law for this reaction is in the. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved rate law determination of the violet Rate Constant K Of Crystal Violet One of these graphs will give a straight line; The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. The chemical rate law consider the. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate. Rate Constant K Of Crystal Violet.
From studylib.net
To determine the rate law for the reaction of crystal violet indicator Rate Constant K Of Crystal Violet The chemical rate law consider the. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. Hydroxylation of crystal violet is: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. One of these graphs will give a straight line; The rate law for this reaction is in. Rate Constant K Of Crystal Violet.
From www.vernier.com
Rate Law Determination of the Crystal Violet Reaction > Experiment 30 Rate Constant K Of Crystal Violet The chemical rate law consider the. What is the differential rate law for the hydroxylation of crystal violet. The rate law for this reaction is in the form: One of these graphs will give a straight line; Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Hydroxylation of crystal violet is: Generally, the rate. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved Expt K302 Rate Law Determination of Crystal Violet Rate Constant K Of Crystal Violet Hydroxylation of crystal violet is: One of these graphs will give a straight line; Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. What is the differential rate law. Rate Constant K Of Crystal Violet.
From www.chegg.com
rate law determination of the crystal violet reaction Rate Constant K Of Crystal Violet Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Hydroxylation of crystal violet is: The chemical rate law consider. Rate Constant K Of Crystal Violet.
From www.youtube.com
Lab 14 Rate Law for Reaction between Crystal Violet and NaOH YouTube Rate Constant K Of Crystal Violet Hydroxylation of crystal violet is: Generally, the rate of the reaction depends on the concentration of one or more of the reactants. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The chemical rate law consider the. The rate law for this reaction is in the form: One of these graphs will give a. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved Rate law Crystal Violet hydroxide reaction 1. How Rate Constant K Of Crystal Violet What is the differential rate law for the hydroxylation of crystal violet. Hydroxylation of crystal violet is: The rate law for this reaction is in the form: Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. Hydrolysis reaction was carried out at varying naoh. Rate Constant K Of Crystal Violet.
From www.chegg.com
Rate law determination of the crystal violet reaction Rate Constant K Of Crystal Violet Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The rate law for this reaction is in the form: The chemical rate law consider the. Hydroxylation of crystal violet is: One of these graphs will give a straight line; What is the differential rate law for the hydroxylation of crystal violet. The exponents n. Rate Constant K Of Crystal Violet.
From www.chegg.com
Rate law determination of the crystal violet reaction Rate Constant K Of Crystal Violet The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. One of these graphs will give a straight line; Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Hydroxylation of crystal violet is: What is the differential. Rate Constant K Of Crystal Violet.
From webapi.bu.edu
⚡ Reaction of crystal violet with naoh. Rate Law Determination of the Rate Constant K Of Crystal Violet The rate law for this reaction is in the form: Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. One of these graphs will give a straight line; What is the differential rate law for the hydroxylation of crystal violet. Hydroxylation of crystal violet. Rate Constant K Of Crystal Violet.
From www.chegg.com
Rate Law Determination of the Crystal Violet Reaction Rate Constant K Of Crystal Violet Generally, the rate of the reaction depends on the concentration of one or more of the reactants. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. The. Rate Constant K Of Crystal Violet.
From webapi.bu.edu
⚡ Reaction of crystal violet with naoh. Rate Law Determination of the Rate Constant K Of Crystal Violet The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The rate law for this reaction is in the form: Hydrolysis reaction was carried out at. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved Assume That The Reaction Order For Hydroxide Ion I... Rate Constant K Of Crystal Violet The chemical rate law consider the. What is the differential rate law for the hydroxylation of crystal violet. One of these graphs will give a straight line; Generally, the rate of the reaction depends on the concentration of one or more of the reactants. Hydroxylation of crystal violet is: Thus, the rate of the hypothetical reaction may. The rate law. Rate Constant K Of Crystal Violet.
From www.youtube.com
Chemical Intro & Theory YouTube Rate Constant K Of Crystal Violet The chemical rate law consider the. What is the differential rate law for the hydroxylation of crystal violet. The rate law for this reaction is in the form: Thus, the rate of the hypothetical reaction may. Hydroxylation of crystal violet is: One of these graphs will give a straight line; The exponents n and m are defined as the order. Rate Constant K Of Crystal Violet.
From studylib.net
A Study Reaction of Crystal Violet with NaOH Learning Rate Constant K Of Crystal Violet One of these graphs will give a straight line; The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Hydroxylation of crystal violet is: What is the differential. Rate Constant K Of Crystal Violet.
From studylib.net
AP Lab of Crystal Violet Rate Constant K Of Crystal Violet Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The rate law for this reaction is in the form: What is the differential rate law for the hydroxylation of crystal violet. The exponents n and m are defined as the order of reaction for. Rate Constant K Of Crystal Violet.
From tukioka-clinic.com
️ Crystal violet and sodium hydroxide reaction order. Rate Law Rate Constant K Of Crystal Violet The chemical rate law consider the. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. One of these graphs will give a straight line; Hydroxylation of crystal violet is: Generally, the rate of the reaction depends on the concentration of one or more of the reactants.. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved Rate Law Determination of the Crystal Violet Reaction Rate Constant K Of Crystal Violet The chemical rate law consider the. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Hydroxylation of crystal violet is: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction. Rate Constant K Of Crystal Violet.
From webapi.bu.edu
⚡ Reaction of crystal violet with naoh. Rate Law Determination of the Rate Constant K Of Crystal Violet Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The chemical rate law consider the. One of these graphs will give a straight line; Hydroxylation of crystal violet is: What is the differential rate law for the hydroxylation of crystal violet. The rate law for this reaction is in the form: Generally, the rate. Rate Constant K Of Crystal Violet.
From www.researchgate.net
Reaction rate constants k' for the dicoloration of crstal violet at Rate Constant K Of Crystal Violet Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The rate law for this reaction is in the form: One of these graphs will give a straight line; The. Rate Constant K Of Crystal Violet.
From webapi.bu.edu
⚡ Reaction of crystal violet with naoh. Rate Law Determination of the Rate Constant K Of Crystal Violet The rate law for this reaction is in the form: The chemical rate law consider the. Hydroxylation of crystal violet is: What is the differential rate law for the hydroxylation of crystal violet. Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The exponents. Rate Constant K Of Crystal Violet.
From www.chegg.com
What is the rate constant k, for this reaction, in Rate Constant K Of Crystal Violet Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The chemical rate law consider the. The rate law for this reaction is in the form: The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular.. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved RATE LAW DETERMINATION OF CRYSTAL VIOLET REACTION Rate Constant K Of Crystal Violet Thus, the rate of the hypothetical reaction may. The rate law for this reaction is in the form: What is the differential rate law for the hydroxylation of crystal violet. Hydroxylation of crystal violet is: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The chemical rate law consider the. One of these graphs. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved PostLab Questions Experiment K302 ate Law Rate Constant K Of Crystal Violet Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. What is the differential rate law for the hydroxylation of crystal violet. One of these graphs will give a straight. Rate Constant K Of Crystal Violet.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K Of Crystal Violet What is the differential rate law for the hydroxylation of crystal violet. Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. The rate law for this reaction. Rate Constant K Of Crystal Violet.
From www.studypool.com
SOLUTION Rate Law Determination Of The Crystal Violet Reaction Studypool Rate Constant K Of Crystal Violet What is the differential rate law for the hydroxylation of crystal violet. Thus, the rate of the hypothetical reaction may. The chemical rate law consider the. Hydroxylation of crystal violet is: One of these graphs will give a straight line; The exponents n and m are defined as the order of reaction for each reactant and k is the rate. Rate Constant K Of Crystal Violet.
From rileighrobertson2017.weebly.com
Crystal Violet Rate Law Lab Rileigh Robertson Rate Constant K Of Crystal Violet The rate law for this reaction is in the form: Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Hydroxylation of crystal violet is: Thus, the rate. Rate Constant K Of Crystal Violet.
From www.chegg.com
Solved Lab for rate law determination of the crystal violet Rate Constant K Of Crystal Violet What is the differential rate law for the hydroxylation of crystal violet. Hydroxylation of crystal violet is: Thus, the rate of the hypothetical reaction may. The rate law for this reaction is in the form: One of these graphs will give a straight line; Hydrolysis reaction was carried out at varying naoh concentrations of 0.008, 0.016 and 0.024 m,. The. Rate Constant K Of Crystal Violet.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant K Of Crystal Violet What is the differential rate law for the hydroxylation of crystal violet. The rate law for this reaction is in the form: Thus, the rate of the hypothetical reaction may. Generally, the rate of the reaction depends on the concentration of one or more of the reactants. The exponents n and m are defined as the order of reaction for. Rate Constant K Of Crystal Violet.
From www.chegg.com
Integrated Rate Laws Crystal Violet Reaction I Rate Constant K Of Crystal Violet Thus, the rate of the hypothetical reaction may. The exponents n and m are defined as the order of reaction for each reactant and k is the rate constant for the reaction at a particular. Hydroxylation of crystal violet is: One of these graphs will give a straight line; What is the differential rate law for the hydroxylation of crystal. Rate Constant K Of Crystal Violet.