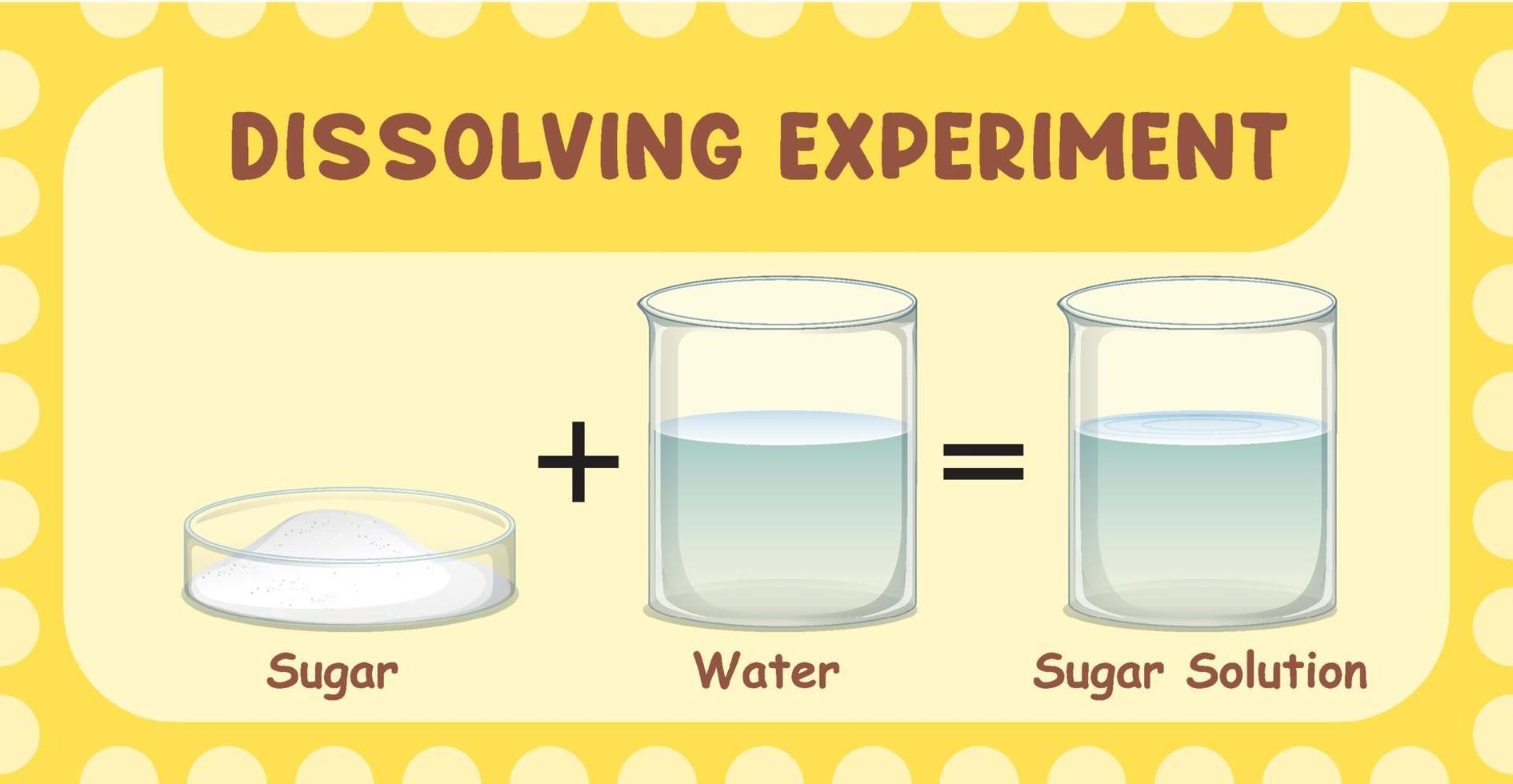
Why Can Salt Dissolve In Water . Water molecules pulling apart the ions (sodium and chloride). Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. The negative areas of the water molecules surround the. When salt is added to water, the positive and negative ions separate. The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. The water molecules surround the ions and pull them. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. Why salt dissolves in water. Have you ever wondered why salt dissolves in water? It’s a fascinating phenomenon that occurs daily in our lives, and yet most of.
from studylibstearine.z21.web.core.windows.net
The positive areas of the water molecules surround the negative chloride ions. Have you ever wondered why salt dissolves in water? The water molecules surround the ions and pull them. Why salt dissolves in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. Water molecules pulling apart the ions (sodium and chloride). When salt is added to water, the positive and negative ions separate. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of.
How Does Water Dissolve Salt
Why Can Salt Dissolve In Water Why salt dissolves in water. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Salt dissolved in water is a rough description of earth's oceans. Have you ever wondered why salt dissolves in water? Why salt dissolves in water. The water molecules surround the ions and pull them. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Water molecules pulling apart the ions (sodium and chloride). The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. When salt is added to water, the positive and negative ions separate. The negative areas of the water molecules surround the. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of.
From geoverse.co.uk
Geoverse Poems on geology, science, Horsham, life, the universe and Why Can Salt Dissolve In Water The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. Salt dissolved in water is a rough description of earth's oceans. The negative. Why Can Salt Dissolve In Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube Why Can Salt Dissolve In Water It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. Why salt dissolves in water. Have you ever wondered why salt dissolves in water? The positive areas of the water molecules surround the negative chloride ions. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in.. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Why Can Salt Dissolve In Water We finally know in detail how salt dissolves in water. Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. The water molecules surround the ions and pull them. Why salt dissolves in water. A machine learning model has revealed. Why Can Salt Dissolve In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Why Can Salt Dissolve In Water At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Why salt dissolves in water. When salt is added to water, the positive and negative ions separate. Salt dissolved in water is a rough description of earth's oceans. Water molecules pulling apart the ions (sodium and chloride). A machine learning model. Why Can Salt Dissolve In Water.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Can Salt Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Why salt dissolves in water. Water molecules pulling apart the ions (sodium and chloride). When salt is added to water, the positive and negative ions separate. At the molecular level, salt dissolves in water due to electrical charges and due. Why Can Salt Dissolve In Water.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Can Salt Dissolve In Water At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Water molecules pulling apart the ions (sodium and chloride). When salt is added to water, the positive and negative ions separate. Why salt dissolves in water. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. In. Why Can Salt Dissolve In Water.
From saylordotorg.github.io
Aqueous Solutions Why Can Salt Dissolve In Water At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. When salt is added to water, the positive and negative ions separate. Why salt dissolves in water. Have you ever wondered why salt dissolves in water? The water molecules surround the ions and pull them. The positive areas of the water. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Can Salt Dissolve In Water Water molecules pulling apart the ions (sodium and chloride). Why salt dissolves in water. We finally know in detail how salt dissolves in water. Have you ever wondered why salt dissolves in water? At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. It’s a fascinating phenomenon that occurs daily in. Why Can Salt Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Why Can Salt Dissolve In Water Salt dissolved in water is a rough description of earth's oceans. The negative areas of the water molecules surround the. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Why salt dissolves in water. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart. Why Can Salt Dissolve In Water.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Why Can Salt Dissolve In Water Salt dissolved in water is a rough description of earth's oceans. The positive areas of the water molecules surround the negative chloride ions. The negative areas of the water molecules surround the. Why salt dissolves in water. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. When salt is added to water, the positive and. Why Can Salt Dissolve In Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Why Can Salt Dissolve In Water The water molecules surround the ions and pull them. The positive areas of the water molecules surround the negative chloride ions. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Why salt dissolves in water.. Why Can Salt Dissolve In Water.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Why Can Salt Dissolve In Water Water molecules pulling apart the ions (sodium and chloride). The negative areas of the water molecules surround the. Why salt dissolves in water. Salt dissolved in water is a rough description of earth's oceans. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. When salt is added to water,. Why Can Salt Dissolve In Water.
From schematicfixtrysted.z22.web.core.windows.net
Diagram Of Salt Dissolving In Water Why Can Salt Dissolve In Water When salt is added to water, the positive and negative ions separate. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. Salt dissolved in water is a rough description of earth's oceans. Why salt dissolves in water. The negative areas of the water molecules surround the. Have you ever wondered why salt dissolves in water?. Why Can Salt Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Why Can Salt Dissolve In Water It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. The negative areas of the water molecules surround the. Salt dissolved in water is a rough description of earth's oceans. Have you ever wondered why salt dissolves in water? A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble. Why Can Salt Dissolve In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Can Salt Dissolve In Water We finally know in detail how salt dissolves in water. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. The negative areas of the water molecules surround the. Water molecules pulling apart the ions (sodium and chloride). When salt is. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Why Can Salt Dissolve In Water Have you ever wondered why salt dissolves in water? It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. Why salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. Water molecules pulling apart the ions (sodium and chloride). When salt is added to water, the positive and negative ions. Why Can Salt Dissolve In Water.
From courses.lumenlearning.com
Water BIO103 Human Biology Why Can Salt Dissolve In Water Water molecules pulling apart the ions (sodium and chloride). Why salt dissolves in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. The negative areas of the water molecules. Why Can Salt Dissolve In Water.
From sciencing.com
What Happens When Salt Is Added to Water? Sciencing Why Can Salt Dissolve In Water In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. When salt is added to water, the positive and negative ions separate. We finally know in detail how salt dissolves in water. At the molecular level, salt dissolves in water due to electrical charges and. Why Can Salt Dissolve In Water.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Why Can Salt Dissolve In Water When salt is added to water, the positive and negative ions separate. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Have you ever wondered why salt dissolves in water? The water molecules surround the ions and pull them. We finally know in detail how salt dissolves in water. Why. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Why Can Salt Dissolve In Water The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Why salt dissolves in water. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. The positive areas of the water molecules surround the negative chloride ions.. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Why Can Salt Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. We finally know in detail how salt dissolves in water. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. Salt dissolved in water is a rough description of earth's oceans. The water molecules surround the. Why Can Salt Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Why Can Salt Dissolve In Water The positive areas of the water molecules surround the negative chloride ions. Water molecules pulling apart the ions (sodium and chloride). The negative areas of the water molecules surround the. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. A machine learning model has. Why Can Salt Dissolve In Water.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Why Can Salt Dissolve In Water It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Why salt dissolves in water. Salt dissolved in water is a rough description of earth's oceans. At. Why Can Salt Dissolve In Water.
From manualwiringbrancher.z14.web.core.windows.net
Diagram Of Salt Dissolving In Water Why Can Salt Dissolve In Water The positive areas of the water molecules surround the negative chloride ions. Why salt dissolves in water. We finally know in detail how salt dissolves in water. When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions (sodium and chloride). In chemistry, it results in a solution, as the ionic bond of. Why Can Salt Dissolve In Water.
From masterconceptsinchemistry.com
How does sodium chloride (NaCl) dissolve in water? Why Can Salt Dissolve In Water Have you ever wondered why salt dissolves in water? Salt dissolved in water is a rough description of earth's oceans. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of. Why Can Salt Dissolve In Water.
From www.numerade.com
SOLVED You notice that NaCl salt easily dissolves in water, but does Why Can Salt Dissolve In Water At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. The negative areas of the water molecules surround the. Have you ever wondered why salt dissolves in water? The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. Water. Why Can Salt Dissolve In Water.
From educatorpages.com
Chemistry Why Can Salt Dissolve In Water It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. Water molecules pulling apart the ions (sodium and chloride). The negative areas of the water molecules surround the. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve. Why Can Salt Dissolve In Water.
From slideplayer.com
Water. ppt download Why Can Salt Dissolve In Water When salt is added to water, the positive and negative ions separate. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. Why salt dissolves in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that. Why Can Salt Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Why Can Salt Dissolve In Water We finally know in detail how salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. It’s a fascinating phenomenon that occurs daily in our lives, and yet most of. Salt dissolved in water is a rough description of earth's oceans. Have you ever wondered why salt dissolves in water? The water molecules surround. Why Can Salt Dissolve In Water.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Why Can Salt Dissolve In Water Water molecules pulling apart the ions (sodium and chloride). In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of. Why salt dissolves in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. The water. Why Can Salt Dissolve In Water.
From curiosityguide.org
How Does Salt Dissolve in Water? Curiosity Guide Why Can Salt Dissolve In Water Have you ever wondered why salt dissolves in water? The negative areas of the water molecules surround the. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Why salt dissolves in water. Salt dissolved in water is a rough description of earth's oceans. The water molecules surround the ions. Why Can Salt Dissolve In Water.
From www.youtube.com
Why Salt Dissolve In Water/உப்பு ஏன் தண்ணீரில் கரைகிறது YouTube Why Can Salt Dissolve In Water We finally know in detail how salt dissolves in water. The negative areas of the water molecules surround the. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. The water. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT SPONCH PowerPoint Presentation, free download ID556676 Why Can Salt Dissolve In Water We finally know in detail how salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. Water molecules pulling apart the ions (sodium and chloride). A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. In chemistry, it results in a solution, as the. Why Can Salt Dissolve In Water.
From bettertogether.org
Is Salt Dissolving In Water A Chemical Change Better Together Why Can Salt Dissolve In Water Salt dissolved in water is a rough description of earth's oceans. Water molecules pulling apart the ions (sodium and chloride). The negative areas of the water molecules surround the. The water molecules surround the ions and pull them. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to. Why Can Salt Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Can Salt Dissolve In Water The water molecules surround the ions and pull them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Water molecules pulling apart the ions (sodium and chloride). The positive areas of the water molecules surround the negative chloride ions. Why salt dissolves in water. Salt dissolved in water is. Why Can Salt Dissolve In Water.