Beer's Law Vernier . The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of a molecule that absorbs light at that wavelength is linear. You will determine the concentration of an. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. To use spectroscopy to prepare a beer’s law plot of. Determining the concentration of a species using a vernier spectrometer. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law.
from www.adda247.com
The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this preliminary activity is to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. The concentration of a molecule that absorbs light at that wavelength is linear. The concentration of an unknown niso4. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. To use spectroscopy to prepare a beer’s law plot of. Determining the concentration of a species using a vernier spectrometer.
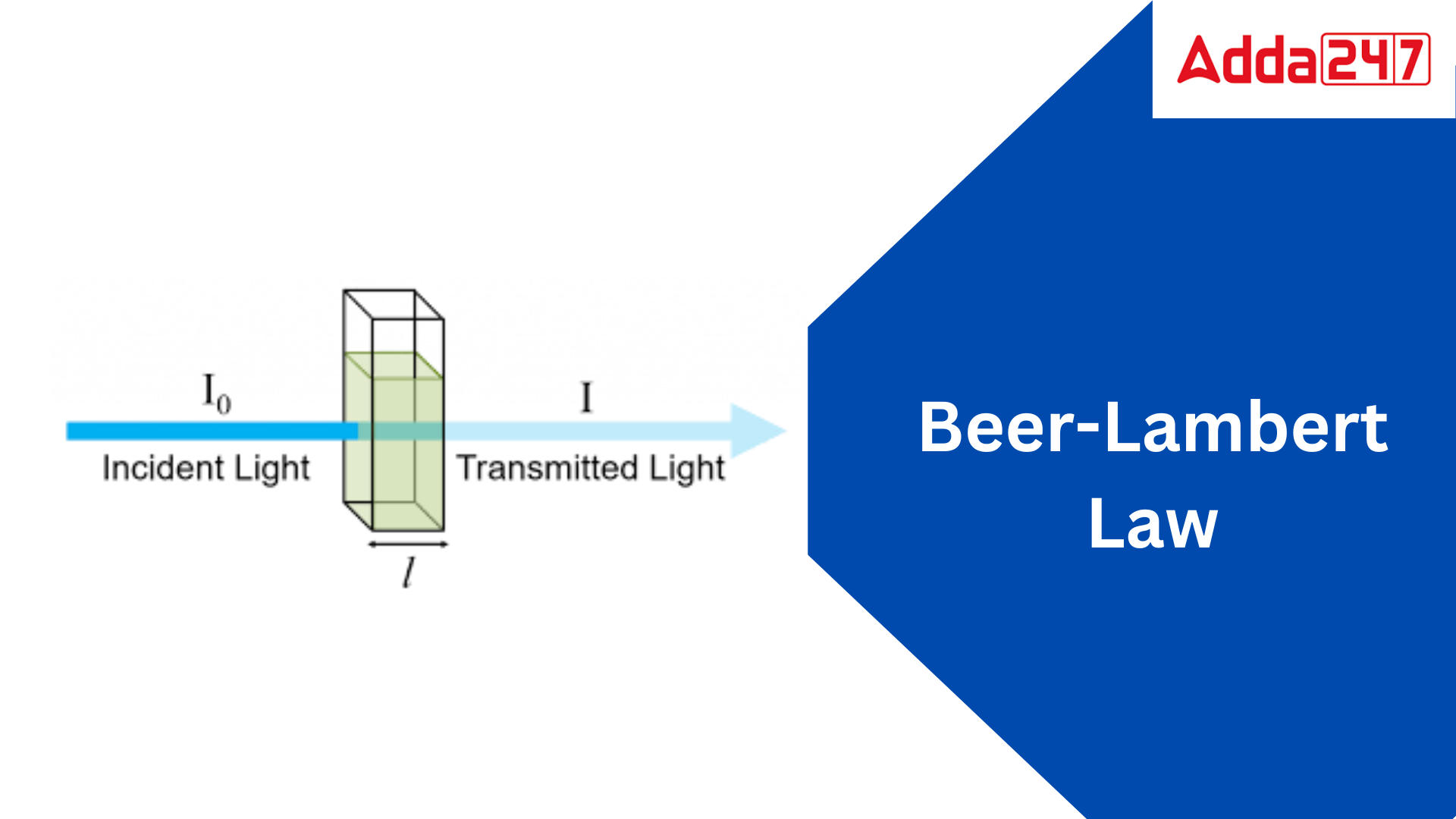
Beer Lambert Law Equation Derivation, Formula, Examples
Beer's Law Vernier The primary objective of this preliminary activity is to determine the concentration of. To use spectroscopy to prepare a beer’s law plot of. Determining the concentration of a species using a vernier spectrometer. The concentration of an unknown niso4. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The concentration of a molecule that absorbs light at that wavelength is linear. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of a molecule that absorbs light at that wavelength is linear. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. Beer's law is followed if a plot of the absorbance at. Beer's Law Vernier.
From www.youtube.com
Spectrophotometer Beer's Law And Colorimetry Lab YouTube Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. You will determine the concentration of an. To use spectroscopy to prepare a beer’s law plot of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. In most experiments, molar absorptivity (ε) and. Beer's Law Vernier.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Vernier You will determine the concentration of an. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law is followed if a plot of. Beer's Law Vernier.
From www.numerade.com
SOLVED Consider the Beer's Law equation A = εbc. Match the variable Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for. Beer's Law Vernier.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The primary objective of this preliminary activity is to determine the concentration of. The. Beer's Law Vernier.
From www.vernier.com
Beer's Law Investigations > Experiment 11 from Investigating Chemistry Beer's Law Vernier The concentration of a molecule that absorbs light at that wavelength is linear. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an. Beer's Law Vernier.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. To use spectroscopy to prepare a beer’s law plot of. Determining the concentration of a species using a vernier spectrometer. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution. Beer's Law Vernier.
From studylib.net
BeerLambert Law using Vernier/Ocean Optics Spectrometer (VSPEC Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. The concentration of a molecule that absorbs light at that wavelength is linear. You will determine the concentration of an. Beer's law is followed. Beer's Law Vernier.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Vernier To use spectroscopy to prepare a beer’s law plot of. The primary objective of this preliminary activity is to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and. Beer's Law Vernier.
From www.slideshare.net
Beers law Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determining the concentration of a species using a vernier spectrometer. The concentration of a molecule that absorbs light at that wavelength is linear. The direct relationship between absorbance and. Beer's Law Vernier.
From chemistrysources.com
قانون بيير (بير) Beer’s Law التعريف و المعادلة مصادر الكيمياء Beer's Law Vernier The concentration of an unknown niso4. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of a molecule that absorbs light at that wavelength is linear. Determining the concentration of a species using a vernier spectrometer. In most experiments,. Beer's Law Vernier.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Vernier Determining the concentration of a species using a vernier spectrometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of an unknown niso4. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The direct relationship between absorbance and concentration for a solution is known as. Beer's Law Vernier.
From brainly.in
Beer lambert law formula Brainly.in Beer's Law Vernier The concentration of an unknown niso4. Determining the concentration of a species using a vernier spectrometer. You will determine the concentration of an. The primary objective of this preliminary activity is to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. To use spectroscopy to prepare a beer’s law. Beer's Law Vernier.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Vernier To use spectroscopy to prepare a beer’s law plot of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this preliminary activity is to determine the concentration of. Determining the concentration of a species. Beer's Law Vernier.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Vernier Determining the concentration of a species using a vernier spectrometer. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The concentration of a molecule that absorbs light at that wavelength is linear. In. Beer's Law Vernier.
From www.researchgate.net
Beer's Law plots for five IR features of amorphous NH 3 . Note that the Beer's Law Vernier Determining the concentration of a species using a vernier spectrometer. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. You will determine the concentration of an. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law.. Beer's Law Vernier.
From www.scribd.com
Lambert Beer Law History Absorbance Radiation Beer's Law Vernier The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. You will determine the concentration of an. The direct. Beer's Law Vernier.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of an unknown niso4. You will determine the concentration of an. Determining the concentration of a species using a vernier spectrometer. The direct relationship between absorbance and. Beer's Law Vernier.
From www.verniercanada.ca
Vernier Spectral Analysis® Vernier Canada Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The concentration of a molecule that absorbs light at that wavelength is linear. The primary objective of this preliminary activity is to determine. Beer's Law Vernier.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. In most experiments, molar absorptivity (ε) and the length. Beer's Law Vernier.
From www.vernier.com
Go Direct® Colorimeter Vernier Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of a molecule that absorbs light at that wavelength is linear. In most experiments, molar absorptivity (ε) and the length (b) are. Beer's Law Vernier.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of a molecule that absorbs light at that wavelength is linear. Determining the. Beer's Law Vernier.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of a molecule that absorbs light at that wavelength is linear. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the. Beer's Law Vernier.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Vernier In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You. Beer's Law Vernier.
From www.vernier.com
Colorimeter Vernier Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the concentration of a species using a vernier spectrometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You will determine the. Beer's Law Vernier.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Vernier You will determine the concentration of an. To use spectroscopy to prepare a beer’s law plot of. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore,. Beer's Law Vernier.
From www.vernier.com
Go Direct® SpectroVis® Plus Spectrophotometer Vernier Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of a molecule that absorbs light at that wavelength is linear. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct. Beer's Law Vernier.
From www.researchgate.net
Adherence to Beer's law Download Scientific Diagram Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the concentration of a species using a vernier spectrometer. Beer's law is followed if a plot of the absorbance at a specific wavelength vs. You will determine the concentration of an. To use spectroscopy to prepare a beer’s law plot of. In most experiments,. Beer's Law Vernier.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the. Beer's Law Vernier.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer's Law Vernier Determining the concentration of a species using a vernier spectrometer. The concentration of a molecule that absorbs light at that wavelength is linear. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. To use spectroscopy to prepare a beer’s law plot of. The direct relationship between absorbance and. Beer's Law Vernier.
From www.softpedia.com
Download Beer's Law Lab 1.02 Beer's Law Vernier Beer's law is followed if a plot of the absorbance at a specific wavelength vs. Determining the concentration of a species using a vernier spectrometer. The concentration of a molecule that absorbs light at that wavelength is linear. The primary objective of this preliminary activity is to determine the concentration of. The direct relationship between absorbance and concentration for a. Beer's Law Vernier.
From www.youtube.com
Lt Chemistry Collection, developed in partnership with Vernier Beer's Beer's Law Vernier The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. The direct relationship between absorbance and concentration for a solution is known as beer’s law. To use spectroscopy to prepare a beer’s law plot of. The direct relationship between absorbance and concentration. Beer's Law Vernier.
From www.youtube.com
BeerLambert Law YouTube Beer's Law Vernier The primary objective of this preliminary activity is to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You will determine the concentration of an. The direct relationship between absorbance and concentration for a solution is known as beer’s law. To use spectroscopy to prepare a beer’s law plot. Beer's Law Vernier.
From www.youtube.com
Beer's Law using a Vernier Go Direct Spectrovis Plus YouTube Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the concentration of a species using a vernier spectrometer. To use spectroscopy to prepare a beer’s law plot of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution. Beer's Law Vernier.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law Vernier The direct relationship between absorbance and concentration for a solution is known as beer’s law. The direct relationship between absorbance and concentration for a solution is known as beer’s law. You will determine the concentration of an. The primary objective of this preliminary activity is to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b). Beer's Law Vernier.