Electrochemistry Of Lemon Battery . In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The electrons create a current as they pass through a solution containing molecules that carry the. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The copper penny that is in contact with the lemon juice is called the electrode of your battery.
from www.comsol.com
The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The electrons create a current as they pass through a solution containing molecules that carry the. The copper penny that is in contact with the lemon juice is called the electrode of your battery. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity.
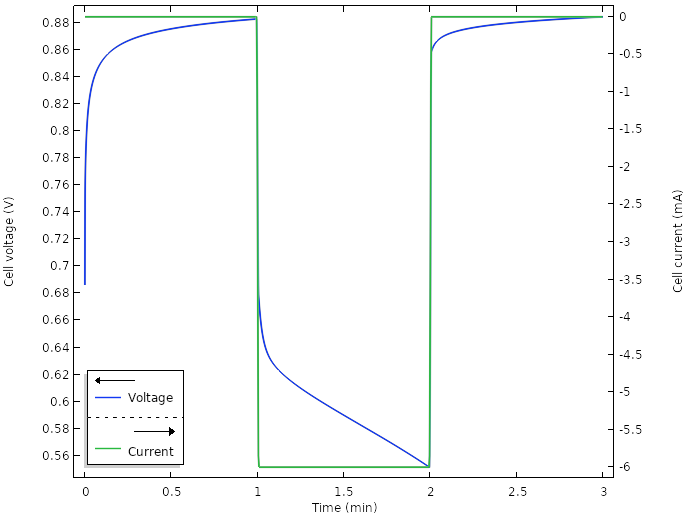
Approaching an Electrochemical Model from Scratch Lemon Battery
Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The electrons create a current as they pass through a solution containing molecules that carry the. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The copper penny that is in contact with the lemon juice is called the electrode of your battery. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb.
From www.freepik.com
Premium Vector Lemon Battery and Multimeter Electrochemical Battery Electrochemistry Of Lemon Battery A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The electrons. Electrochemistry Of Lemon Battery.
From teachbesideme.com
Lemon Battery Experiment Teach Beside Me Electrochemistry Of Lemon Battery The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The electrons create a current as they pass through a solution containing molecules that carry the. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper. Electrochemistry Of Lemon Battery.
From en.wikipedia.org
Lemon battery Wikipedia Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The electrons create a current as they pass through a solution containing molecules that carry the. The battery you just made has a copper and an aluminum. Electrochemistry Of Lemon Battery.
From www.youtube.com
Electrochemical Lemon Battery YouTube Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The copper penny that is in contact with the lemon juice is called the electrode of your battery. The electrons create a current as they pass through a solution containing molecules that carry the. The battery you just made has a copper. Electrochemistry Of Lemon Battery.
From www.youtube.com
Recharge Your Lesson Plan! Moving Beyond the Lemon Battery to Teach Electrochemistry Of Lemon Battery The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The copper penny that is in contact with the lemon juice is called the electrode of your. Electrochemistry Of Lemon Battery.
From www.fizzicseducation.com.au
A Simple Lemon Battery Fizzics Education Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb.. Electrochemistry Of Lemon Battery.
From studylib.net
2A The lemon battery Electrochemistry Of Lemon Battery A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice.. Electrochemistry Of Lemon Battery.
From www.youtube.com
Lemon Battery Science Project How to glow LED with Lemons YouTube Electrochemistry Of Lemon Battery The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The copper penny that is in contact with the lemon juice is called the electrode of your battery.. Electrochemistry Of Lemon Battery.
From www.youtube.com
How to Make a Lemon Battery YouTube Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The electrons create a current as they pass through a solution containing molecules that carry the. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. The. Electrochemistry Of Lemon Battery.
From sciencenotes.org
Lemon Battery Experiment Electrochemistry Of Lemon Battery The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The copper penny that is in contact with the lemon juice is called the electrode of your battery. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon. Electrochemistry Of Lemon Battery.
From elecengworld1.blogspot.com
How to make Lemon Battery? Electrical Engineering Blog Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. The copper. Electrochemistry Of Lemon Battery.
From www.youtube.com
Electrochemistry Lemon Battery pt1/3 (no captions) YouTube Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. In our. Electrochemistry Of Lemon Battery.
From kitchenpantryscientist.com
Lemon Batteries « The Kitchen Pantry Scientist Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice.. Electrochemistry Of Lemon Battery.
From www.freepik.com
Premium Vector Lemon Battery and LED Electrochemical Battery Electrochemistry Of Lemon Battery The copper penny that is in contact with the lemon juice is called the electrode of your battery. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. In our lemon battery, the copper plate is our. Electrochemistry Of Lemon Battery.
From www.researchgate.net
(PDF) Construction and evaluation of electrical properties of a lemon Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The electrons create a current as they pass through a solution containing molecules that carry the. The battery produces enough electricity to power an led or other. Electrochemistry Of Lemon Battery.
From melscience.com
Lemon battery MEL Chemistry Electrochemistry Of Lemon Battery The copper penny that is in contact with the lemon juice is called the electrode of your battery. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The electrons create a current as they pass through. Electrochemistry Of Lemon Battery.
From electrical-engineering-world1.blogspot.com
Electrical Engineering World How to make Lemon Battery Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The copper penny that is in contact with the lemon juice is called the electrode of your battery. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light. Electrochemistry Of Lemon Battery.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Electrochemistry Of Lemon Battery The electrons create a current as they pass through a solution containing molecules that carry the. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The copper. Electrochemistry Of Lemon Battery.
From www.comsol.com
Approaching an Electrochemical Model from Scratch Lemon Battery Electrochemistry Of Lemon Battery The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The electrons create a current as they pass through a solution containing molecules that carry the. The copper penny that is in contact with the lemon juice is called the electrode of your battery. A. Electrochemistry Of Lemon Battery.
From www.youtube.com
Electrochemistry Lemon Battery pt3/3 (no captions) YouTube Electrochemistry Of Lemon Battery A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. Part of the penny should be in contact with the lemon juice, as a chemical reaction between. Electrochemistry Of Lemon Battery.
From sciencenotes.org
Lemon Battery Experiment Electrochemistry Of Lemon Battery The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The copper penny that is in contact with the lemon juice is called the electrode of your battery. Part of the penny should be in. Electrochemistry Of Lemon Battery.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2351686 Electrochemistry Of Lemon Battery A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. Part of the penny should be in contact with the. Electrochemistry Of Lemon Battery.
From melscience.com
Lemon battery MEL Chemistry Electrochemistry Of Lemon Battery The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. The copper penny that is in contact with the lemon juice is called the electrode of your battery.. Electrochemistry Of Lemon Battery.
From www.thoughtco.com
How to Make a Fruit Battery Electrochemistry Of Lemon Battery The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The copper penny that is in contact with the lemon juice is called the electrode of your battery. The electrons create a current as they pass through a solution containing molecules that carry the. Part of the penny should be in contact with. Electrochemistry Of Lemon Battery.
From www.shutterstock.com
Batterie lemon plus de 1 illustrations et dessins de stock libres de Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. In our lemon battery, the copper plate is our positive. Electrochemistry Of Lemon Battery.
From printablezonetipula.z21.web.core.windows.net
Fruit Battery Science Experiment Report Electrochemistry Of Lemon Battery The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The copper penny that is in contact with the lemon juice is called the electrode of your battery. The electrons create a current as they. Electrochemistry Of Lemon Battery.
From www.slideserve.com
PPT Lemon Battery!!! PowerPoint Presentation, free download ID2711861 Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The copper. Electrochemistry Of Lemon Battery.
From www.npr.org
The Science Behind The Lemon Battery Short Wave NPR Electrochemistry Of Lemon Battery In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. A chemical reaction between the copper and zinc plates and the citric acid produces a small current,. Electrochemistry Of Lemon Battery.
From ar.inspiredpencil.com
Lemon Experiment Electrochemistry Of Lemon Battery The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The copper penny that is in contact with the lemon juice is called the electrode of your battery. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative anode. Part of the penny should be in. Electrochemistry Of Lemon Battery.
From www.youtube.com
Electrochemistry Lemon Battery pt2/3 (no captions) YouTube Electrochemistry Of Lemon Battery The copper penny that is in contact with the lemon juice is called the electrode of your battery. The electrons create a current as they pass through a solution containing molecules that carry the. A chemical reaction between the copper and zinc plates and the citric acid produces a small current, that is able to power a light bulb. The. Electrochemistry Of Lemon Battery.
From www.youtube.com
How To Make Lemon Battery At Home Lemon Battery Science Experiment Electrochemistry Of Lemon Battery The electrons create a current as they pass through a solution containing molecules that carry the. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The copper penny that is in contact with the lemon juice. Electrochemistry Of Lemon Battery.
From www.slideserve.com
PPT Electrochemical Cells (Batteries) PowerPoint Presentation, free Electrochemistry Of Lemon Battery The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. The copper penny that is in contact with the lemon juice is called the electrode of your battery. In our lemon battery, the copper plate is our positive cathode and the zinc plate the negative. Electrochemistry Of Lemon Battery.
From www.shutterstock.com
How Make Simple Lemon Battery Educational Stok İllüstrasyon 2085208819 Electrochemistry Of Lemon Battery Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. The battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The battery produces enough electricity to power an led or. Electrochemistry Of Lemon Battery.
From www.comsol.com
Approaching an Electrochemical Model from Scratch Lemon Battery Electrochemistry Of Lemon Battery The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in generating electricity. In our. Electrochemistry Of Lemon Battery.
From www.comsol.com
Approaching an Electrochemical Model from Scratch Lemon Battery Electrochemistry Of Lemon Battery The electrons create a current as they pass through a solution containing molecules that carry the. The battery produces enough electricity to power an led or other small device, but not enough to cause harm, even if you touch both electrodes. Part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper. Electrochemistry Of Lemon Battery.