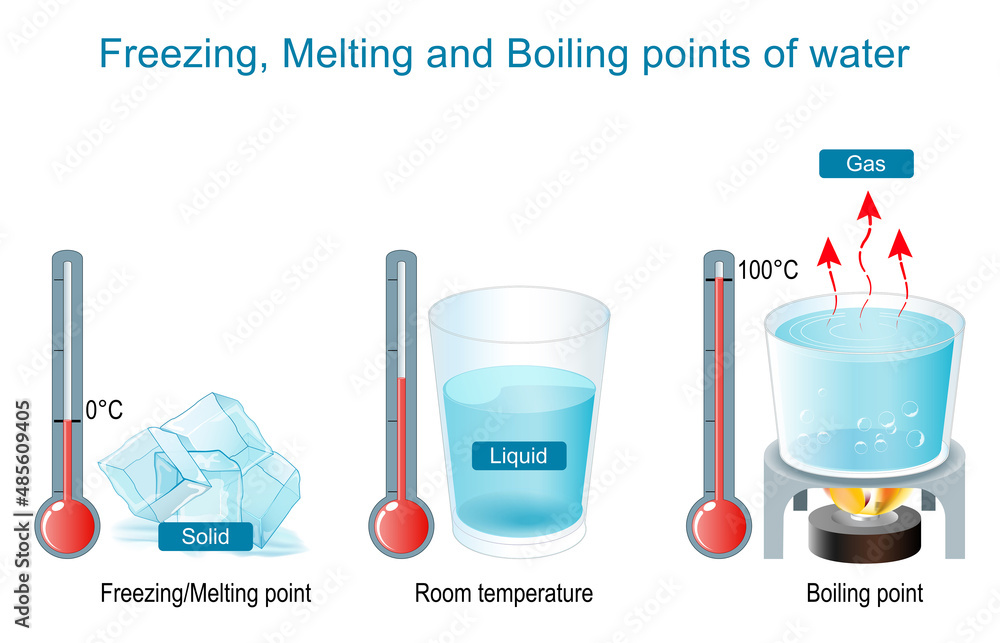
Where Is The Boiling Point Of Pure Water . Water that contains impurities (such as salted water) boils at a higher temperature than pure water. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). Normally when we boil a liquid, we do so at atmospheric pressure. At other elevations the melting point will change due to different. The standard boiling point of water is 99.61 °c at 1 bar of pressure. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The boiling point of a liquid varies according to the applied pressure; From the highest land point above sea level, mount. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar.
from stock.adobe.com
The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The boiling point of a liquid varies according to the applied pressure; From the highest land point above sea level, mount. At other elevations the melting point will change due to different. Normally when we boil a liquid, we do so at atmospheric pressure. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The standard boiling point of water is 99.61 °c at 1 bar of pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation).
Boiling and Evaporation, Freezing and Melting Points of Water. Stock
Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. At other elevations the melting point will change due to different. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. From the highest land point above sea level, mount. The boiling point of a liquid varies according to the applied pressure; Normally when we boil a liquid, we do so at atmospheric pressure. Water that contains impurities (such as salted water) boils at a higher temperature than pure water. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). The standard boiling point of water is 99.61 °c at 1 bar of pressure. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid.
From www.numerade.com
Arrange the boiling points of the aqueous solutions, relative to pure Where Is The Boiling Point Of Pure Water Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c at 1 bar of pressure. The standard boiling. Where Is The Boiling Point Of Pure Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Where Is The Boiling Point Of Pure Water The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). The boiling point of a liquid varies according to the applied pressure; Water that contains impurities (such as salted water) boils at a higher temperature than pure water. The normal boiling point or the atmospheric boiling point is the. Where Is The Boiling Point Of Pure Water.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Where Is The Boiling Point Of Pure Water The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The melting point of water is about 32 degrees fahrenheit (0. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved The boiling point of water is 100.00∘C at 1 Where Is The Boiling Point Of Pure Water The boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c at 1 bar of pressure. Water that contains impurities (such as salted water) boils at a higher temperature than pure water. Normally when we boil a liquid, we do so at atmospheric pressure. At other elevations the melting point. Where Is The Boiling Point Of Pure Water.
From pakmcqs.com
Boiling point of pure water is __________? PakMcqs Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Here, we take a look at the boiling points of water at a variety of locations, as. Where Is The Boiling Point Of Pure Water.
From www.numerade.com
SOLVED Texts Arrange the boiling points of the aqueous solutions Where Is The Boiling Point Of Pure Water Water that contains impurities (such as salted water) boils at a higher temperature than pure water. At other elevations the melting point will change due to different. The boiling point of a liquid varies according to the applied pressure; The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation).. Where Is The Boiling Point Of Pure Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Where Is The Boiling Point Of Pure Water At other elevations the melting point will change due to different. Water that contains impurities (such as salted water) boils at a higher temperature than pure water. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c. Where Is The Boiling Point Of Pure Water.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Where Is The Boiling Point Of Pure Water The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Water that contains impurities (such as salted water) boils at a higher temperature than. Where Is The Boiling Point Of Pure Water.
From slideplayer.com
Physical and Chemical properties ppt download Where Is The Boiling Point Of Pure Water The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The boiling point of a liquid varies according to the applied pressure; Water that contains impurities (such as salted water) boils at a higher temperature than pure water. If this pressure is the standard pressure of 1 atm (101.3. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved 1. Determine the boiling point of pure water given Where Is The Boiling Point Of Pure Water Water that contains impurities (such as salted water) boils at a higher temperature than pure water. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Here, we take a look at the boiling points of water at a variety of locations, as well as the. Where Is The Boiling Point Of Pure Water.
From www.numerade.com
SOLVEDIn a mountainous location, the boiling point of pure water is Where Is The Boiling Point Of Pure Water From the highest land point above sea level, mount. Normally when we boil a liquid, we do so at atmospheric pressure. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The standard boiling point of water is 99.61 °c at 1 bar of pressure.. Where Is The Boiling Point Of Pure Water.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. Where Is The Boiling Point Of Pure Water At other elevations the melting point will change due to different. The standard boiling point of water is 99.61 °c at 1 bar of pressure. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Normally when we boil a liquid, we do so at atmospheric. Where Is The Boiling Point Of Pure Water.
From www.thoughtco.com
What Is the Boiling Point of Water? Where Is The Boiling Point Of Pure Water The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The standard boiling point, as defined by the iupac in 1982, is the temperature. Where Is The Boiling Point Of Pure Water.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. From the highest land point above sea level, mount. The standard boiling point of water is 99.61 °c at 1 bar of pressure. The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure. Where Is The Boiling Point Of Pure Water.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 Where Is The Boiling Point Of Pure Water From the highest land point above sea level, mount. The boiling point of a liquid varies according to the applied pressure; Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Water that contains impurities (such as salted water) boils at a higher temperature than. Where Is The Boiling Point Of Pure Water.
From www.numerade.com
SOLVED C. BoilingPoint Elevation Table 10.6 (data) Boiling point of Where Is The Boiling Point Of Pure Water The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. Normally when we boil a liquid, we do so at atmospheric pressure. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The standard. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved The boiling point of pure water is 100 °C. The Where Is The Boiling Point Of Pure Water The standard boiling point of water is 99.61 °c at 1 bar of pressure. From the highest land point above sea level, mount. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius). Where Is The Boiling Point Of Pure Water.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. At other elevations the melting point will change due to different. The normal boiling point or the. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, Where Is The Boiling Point Of Pure Water Water that contains impurities (such as salted water) boils at a higher temperature than pure water. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. At other elevations the melting point will change due to different. The standard boiling point of water is 99.61 °c. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. At other elevations the melting point will change due to different. From the highest land point above sea level, mount. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure. Where Is The Boiling Point Of Pure Water.
From brainly.com
Arrange the boiling points of the aqueous solutions, relative to pure Where Is The Boiling Point Of Pure Water The standard boiling point of water is 99.61 °c at 1 bar of pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. At other elevations the melting point will change due to different. The standard boiling point,. Where Is The Boiling Point Of Pure Water.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Where Is The Boiling Point Of Pure Water Normally when we boil a liquid, we do so at atmospheric pressure. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The standard boiling point, as defined by the. Where Is The Boiling Point Of Pure Water.
From www.youtube.com
COMPARING BOILING POINTS OF PURE SUBSTANCE YouTube Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Here, we take a look at the boiling points of water at a variety of locations, as. Where Is The Boiling Point Of Pure Water.
From slideplayer.com
Heterogeneous vs. Homogeneous ppt download Where Is The Boiling Point Of Pure Water The boiling point of a liquid varies according to the applied pressure; At other elevations the melting point will change due to different. Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The normal boiling point or the. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved The table below shows the boiling points of pure Where Is The Boiling Point Of Pure Water At other elevations the melting point will change due to different. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. If this pressure is. Where Is The Boiling Point Of Pure Water.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Where Is The Boiling Point Of Pure Water The boiling point of a liquid varies according to the applied pressure; At other elevations the melting point will change due to different. Water that contains impurities (such as salted water) boils at a higher temperature than pure water. The standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure. Where Is The Boiling Point Of Pure Water.
From www.chegg.com
Solved suppose the boiling point of pure water at h h Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. From the highest land point above sea level, mount. At other elevations the melting point will change due to different. The standard boiling point of water is 99.61 °c at 1 bar of pressure. Water that contains impurities (such as salted. Where Is The Boiling Point Of Pure Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Where Is The Boiling Point Of Pure Water At other elevations the melting point will change due to different. Water that contains impurities (such as salted water) boils at a higher temperature than pure water. Normally when we boil a liquid, we do so at atmospheric pressure. From the highest land point above sea level, mount. Here, we take a look at the boiling points of water at. Where Is The Boiling Point Of Pure Water.
From www.slideshare.net
Purification Of Substances 1 Where Is The Boiling Point Of Pure Water The boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c at 1 bar of pressure. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. The normal boiling point or the atmospheric boiling point. Where Is The Boiling Point Of Pure Water.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation ID5410728 Where Is The Boiling Point Of Pure Water The standard boiling point of water is 99.61 °c at 1 bar of pressure. The boiling point of a liquid varies according to the applied pressure; The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The melting point of water is about 32 degrees fahrenheit (0 degrees celsius). Where Is The Boiling Point Of Pure Water.
From www.toppr.com
(a) The boiling point of pure water is 373K. Calculate the boiling Where Is The Boiling Point Of Pure Water The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. The boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c at 1 bar of pressure. Water that contains impurities (such as salted water) boils at a higher temperature. Where Is The Boiling Point Of Pure Water.
From slideplayer.com
First Six Weeks Vocabulary ppt download Where Is The Boiling Point Of Pure Water The boiling point of a liquid varies according to the applied pressure; The melting point of water is about 32 degrees fahrenheit (0 degrees celsius) for pure water at sea level (normal elevation). Water that contains impurities (such as salted water) boils at a higher temperature than pure water. Normally when we boil a liquid, we do so at atmospheric. Where Is The Boiling Point Of Pure Water.
From www.slideserve.com
PPT Opening Assignment PowerPoint Presentation, free download ID Where Is The Boiling Point Of Pure Water Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. From the highest land point above sea level, mount. The boiling point of a liquid varies according to the applied pressure; At other elevations the melting point will change due to different. The standard boiling. Where Is The Boiling Point Of Pure Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Where Is The Boiling Point Of Pure Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. The standard boiling point of water is 99.61 °c at 1 bar of pressure. Water that contains impurities (such as salted water) boils at a higher temperature than pure. Where Is The Boiling Point Of Pure Water.
From www.solutionspile.com
[Solved] Arrange the boiling points of the aqueous soluti Where Is The Boiling Point Of Pure Water The normal boiling point or the atmospheric boiling point is the boiling point at 1 atmosphere of pressure or sea level. From the highest land point above sea level, mount. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. If this pressure is the. Where Is The Boiling Point Of Pure Water.