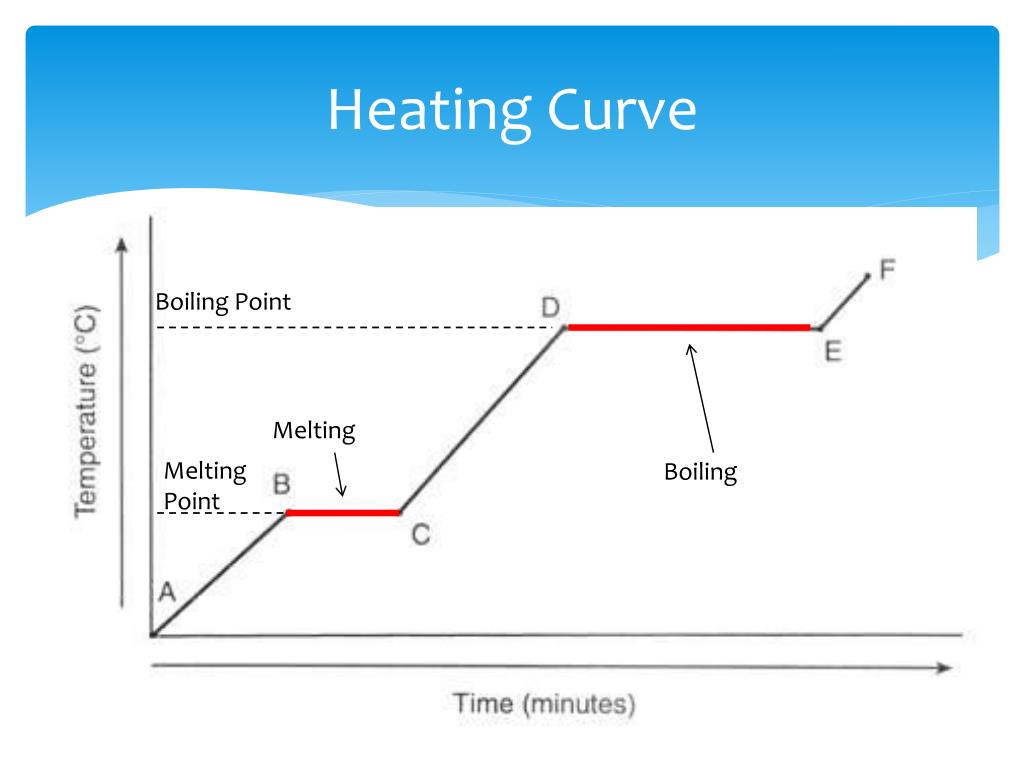
Define Heating Or Cooling Curve . For heating curves, we start with a solid and add heat energy. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. The ice is in a closed container. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. List the changes of state. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. It can be used to determine the melting point. Heating and cooling curves (also called temperature curves) | chem101 online: They show how the temperature changes as a substance is. Steeper slopes indicate a higher heat transfer rate,. The slope of the heating curve represents the rate at which the substance absorbs heat. As heat is steadily added to the. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. Relate the change of state to a. Heating and cooling curves are graphs.
from www.slideserve.com
The slope of the heating curve represents the rate at which the substance absorbs heat. As heat is steadily added to the. Relate the change of state to a. The ice is in a closed container. Heating curves show how the temperature changes as a substance is heated up. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating and cooling curves are graphs. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Heating curves relate temperature changes to phase transitions. They show how the temperature changes as a substance is.
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation
Define Heating Or Cooling Curve Steeper slopes indicate a higher heat transfer rate,. List the changes of state. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. They show how the temperature changes as a substance is. The ice is in a closed container. As heat is steadily added to the. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. Relate the change of state to a. Heating and cooling curves (also called temperature curves) | chem101 online: Heating and cooling curves are graphs. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. It can be used to determine the melting point. Steeper slopes indicate a higher heat transfer rate,.
From martindxmguide.blogspot.com
36 Chemistry Heating Curve Worksheet support worksheet Define Heating Or Cooling Curve Heating curves show how the temperature changes as a substance is heated up. The ice is in a closed container. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. As heat is steadily added to the. They show how the temperature changes as a substance is. Imagine that you. Define Heating Or Cooling Curve.
From www.worldwisetutoring.com
Heating and Cooling Curves WorldWise Tutoring Define Heating Or Cooling Curve Heating and cooling curves are graphs. The slope of the heating curve represents the rate at which the substance absorbs heat. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. As heat is steadily added to the. The ice is in a closed container. Heating. Define Heating Or Cooling Curve.
From obropolox.blogspot.com
39 heating and cooling curves worksheet Worksheet Resource Define Heating Or Cooling Curve Cooling curves are the opposite. Heating curves relate temperature changes to phase transitions. Steeper slopes indicate a higher heat transfer rate,. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Relate the change of state to a. As heat is steadily added to the. A cooling curve is a. Define Heating Or Cooling Curve.
From studylib.net
HEATING AND COOLING CURVE Define Heating Or Cooling Curve Heating curves relate temperature changes to phase transitions. The slope of the heating curve represents the rate at which the substance absorbs heat. Steeper slopes indicate a higher heat transfer rate,. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. List the changes of state. It can be used. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves of Water PowerPoint Presentation, free Define Heating Or Cooling Curve Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The ice is in a closed container. For heating curves, we start with a solid and add heat energy. Heating curves relate temperature changes to phase transitions. A superheated liquid, a liquid at a temperature and. Define Heating Or Cooling Curve.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Define Heating Or Cooling Curve A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Cooling curves are the opposite. The slope of the heating curve represents the rate at which the substance absorbs heat. Heating and cooling curves (also called temperature curves) | chem101 online: List the changes of state. As heat is steadily. Define Heating Or Cooling Curve.
From www.researchgate.net
3) Cooling curve for a pure metal. Download Scientific Diagram Define Heating Or Cooling Curve Heating curves relate temperature changes to phase transitions. Cooling curves are the opposite. Heating curves show how the temperature changes as a substance is heated up. Steeper slopes indicate a higher heat transfer rate,. Relate the change of state to a. For heating curves, we start with a solid and add heat energy. They show how the temperature changes as. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Latent Heat PowerPoint Presentation, free download ID1378874 Define Heating Or Cooling Curve They show how the temperature changes as a substance is. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. Heating curves show how the temperature changes as a substance is heated up. List the changes of state. Heating and cooling curves. Define Heating Or Cooling Curve.
From www.youtube.com
Cooling curve YouTube Define Heating Or Cooling Curve Cooling curves are the opposite. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating and cooling curves are graphs. Relate the change of state to a. As heat is steadily added to the. For heating curves, we start with a solid and add heat. Define Heating Or Cooling Curve.
From www.youtube.com
How to Read a Cooling Curve YouTube Define Heating Or Cooling Curve Steeper slopes indicate a higher heat transfer rate,. Heating curves relate temperature changes to phase transitions. List the changes of state. For heating curves, we start with a solid and add heat energy. They show how the temperature changes as a substance is. A cooling curve is a line graph that represents the change of phase of matter, typically from. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Phase Changes and Heat PowerPoint Presentation, free download Define Heating Or Cooling Curve They show how the temperature changes as a substance is. Steeper slopes indicate a higher heat transfer rate,. Cooling curves are the opposite. The slope of the heating curve represents the rate at which the substance absorbs heat. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its. Define Heating Or Cooling Curve.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Define Heating Or Cooling Curve The slope of the heating curve represents the rate at which the substance absorbs heat. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Define Heating Or Cooling Curve As heat is steadily added to the. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Steeper slopes indicate a higher heat transfer rate,. The ice is in a closed container. A cooling curve is a line graph that represents the change of phase of. Define Heating Or Cooling Curve.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Define Heating Or Cooling Curve Heating curves show how the temperature changes as a substance is heated up. Heating and cooling curves are graphs. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. Steeper slopes indicate a higher heat transfer rate,. The slope of the heating curve represents the rate at which the substance. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Define Heating Or Cooling Curve List the changes of state. Heating and cooling curves are graphs. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Relate the change. Define Heating Or Cooling Curve.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Define Heating Or Cooling Curve Heating curves relate temperature changes to phase transitions. For heating curves, we start with a solid and add heat energy. As heat is steadily added to the. The ice is in a closed container. Steeper slopes indicate a higher heat transfer rate,. Relate the change of state to a. List the changes of state. Heating and cooling curves are graphs.. Define Heating Or Cooling Curve.
From quizlet.com
heating/cooling curve Diagram Quizlet Define Heating Or Cooling Curve The slope of the heating curve represents the rate at which the substance absorbs heat. It can be used to determine the melting point. Heating curves relate temperature changes to phase transitions. Heating curves show how the temperature changes as a substance is heated up. A cooling curve is a line graph that represents the change of phase of matter,. Define Heating Or Cooling Curve.
From www.pinterest.com
Heating Curves Quizzes and answers, Biology worksheet, Answers Define Heating Or Cooling Curve The slope of the heating curve represents the rate at which the substance absorbs heat. The ice is in a closed container. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. As heat is steadily added to the. It can be used to determine the melting point. They show how. Define Heating Or Cooling Curve.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Define Heating Or Cooling Curve The slope of the heating curve represents the rate at which the substance absorbs heat. It can be used to determine the melting point. For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is. Define Heating Or Cooling Curve.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Define Heating Or Cooling Curve Heating and cooling curves (also called temperature curves) | chem101 online: For heating curves, we start with a solid and add heat energy. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. Steeper slopes indicate a higher heat transfer rate,. The ice is in a closed container. Cooling curves are. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Define Heating Or Cooling Curve Steeper slopes indicate a higher heat transfer rate,. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. As heat is steadily added to the. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. For. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Thermodynamics Heating/Cooling Curves PowerPoint Presentation Define Heating Or Cooling Curve The ice is in a closed container. The slope of the heating curve represents the rate at which the substance absorbs heat. Relate the change of state to a. Heating and cooling curves are graphs. List the changes of state. Heating and cooling curves (also called temperature curves) | chem101 online: As heat is steadily added to the. Heating curves. Define Heating Or Cooling Curve.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Define Heating Or Cooling Curve As heat is steadily added to the. Heating and cooling curves are graphs. Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Steeper slopes indicate a higher heat transfer rate,. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Define Heating Or Cooling Curve As heat is steadily added to the. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. The ice is in a closed container. Heating and cooling curves (also called temperature curves) | chem101 online: The slope of the heating curve represents the rate at which the substance absorbs heat.. Define Heating Or Cooling Curve.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Define Heating Or Cooling Curve List the changes of state. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. A superheated liquid,. Define Heating Or Cooling Curve.
From www.e-streetlight.com
Heating And Cooling Curve Worksheet E Street Light Define Heating Or Cooling Curve Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating and cooling curves (also called temperature curves) | chem101 online: List the changes of state. As heat is steadily added to the. Heating and cooling curves are graphs. Cooling curves are the opposite. A cooling. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Define Heating Or Cooling Curve The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. List the changes of state. It can be used to determine the melting point. For heating curves, we start with a solid and add heat energy. As heat is steadily added to the. A superheated liquid, a liquid at a temperature. Define Heating Or Cooling Curve.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Define Heating Or Cooling Curve Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. For heating curves, we start with a solid and add heat energy. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating and cooling curves (also called. Define Heating Or Cooling Curve.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Define Heating Or Cooling Curve As heat is steadily added to the. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. It can be used to determine the melting point. A superheated liquid, a liquid at a temperature and pressure at which it should be a. Define Heating Or Cooling Curve.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Define Heating Or Cooling Curve A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. Cooling curves are the opposite. List the changes of state. They show how the temperature changes as a substance is. It can be used to determine the melting point. The heating curve. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Define Heating Or Cooling Curve List the changes of state. For heating curves, we start with a solid and add heat energy. The ice is in a closed container. Heating curves show how the temperature changes as a substance is heated up. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting. Define Heating Or Cooling Curve.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion Define Heating Or Cooling Curve The ice is in a closed container. They show how the temperature changes as a substance is. Steeper slopes indicate a higher heat transfer rate,. It can be used to determine the melting point. A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. The slope of the heating curve. Define Heating Or Cooling Curve.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Define Heating Or Cooling Curve They show how the temperature changes as a substance is. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. List the changes of state. The slope of the heating curve represents the rate at which the substance absorbs heat. Heating curves show how the temperature changes as a substance is. Define Heating Or Cooling Curve.
From www.jove.com
Heating and Cooling Curves JoVE Wordpress Development Define Heating Or Cooling Curve Steeper slopes indicate a higher heat transfer rate,. The ice is in a closed container. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The heating curve is a graphical representation of the correlation between heat input and the temperature of a substance. Heating and. Define Heating Or Cooling Curve.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Define Heating Or Cooling Curve A superheated liquid, a liquid at a temperature and pressure at which it should be a gas, is not stable. A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. The heating curve is a graphical representation of the correlation between heat. Define Heating Or Cooling Curve.