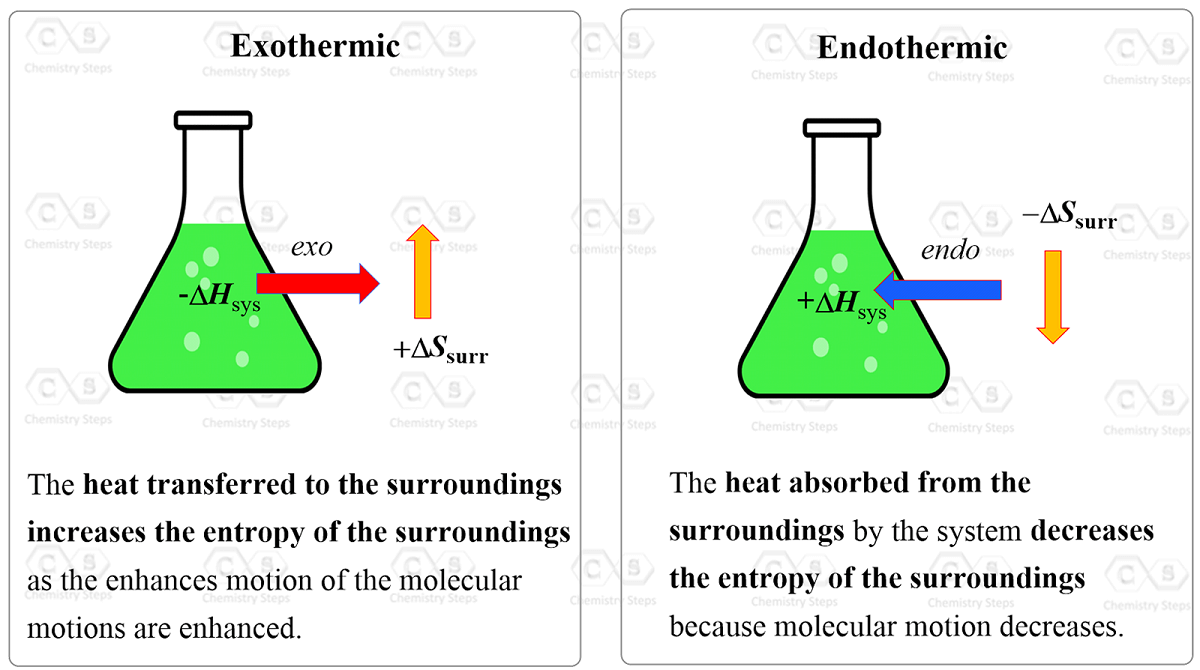
Endothermic Reaction With Negative Entropy Change . This is an endothermic reaction with a negative entropy change. To calculate entropy changes for a chemical reaction. To summarize, we can say that. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Ultimately, all molecules decompose to their. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. Overall, the entropy of the. What matters is the total entropy change, which is the sum of the entropy changes of. We have seen that the energy given off (or absorbed) by a reaction,.
from ar.inspiredpencil.com
Overall, the entropy of the. To summarize, we can say that. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. This is an endothermic reaction with a negative entropy change. What matters is the total entropy change, which is the sum of the entropy changes of. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. Ultimately, all molecules decompose to their. To calculate entropy changes for a chemical reaction.
Entropy Definition Chemistry
Endothermic Reaction With Negative Entropy Change What matters is the total entropy change, which is the sum of the entropy changes of. To summarize, we can say that. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. Overall, the entropy of the. Ultimately, all molecules decompose to their. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. What matters is the total entropy change, which is the sum of the entropy changes of. This is an endothermic reaction with a negative entropy change. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. We have seen that the energy given off (or absorbed) by a reaction,. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. To calculate entropy changes for a chemical reaction.
From sciencenotes.org
What Is Entropy? Definition and Examples Endothermic Reaction With Negative Entropy Change This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. To summarize,. Endothermic Reaction With Negative Entropy Change.
From opentextbc.ca
Measuring Entropy and Entropy Changes Introductory Chemistry 1st Endothermic Reaction With Negative Entropy Change An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. To calculate entropy changes for a chemical reaction. Ultimately, all molecules decompose to their. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. The reactions are spontaneous when the entropy and enthalpy are negative. Endothermic Reaction With Negative Entropy Change.
From byjus.com
67. S=qsquareT. Where S is entropy . so in case of endothermic Endothermic Reaction With Negative Entropy Change Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. What matters. Endothermic Reaction With Negative Entropy Change.
From general.chemistrysteps.com
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps Endothermic Reaction With Negative Entropy Change Overall, the entropy of the. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. What matters is the total entropy change, which is the sum of the entropy. Endothermic Reaction With Negative Entropy Change.
From www.chegg.com
Solved 11 of 25 Part A What can be said about an endothermic Endothermic Reaction With Negative Entropy Change This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. This is an endothermic reaction with a negative entropy change. To summarize, we can say that. To calculate entropy changes for a chemical reaction. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the. Endothermic Reaction With Negative Entropy Change.
From slideplayer.com
Spontaneity, Entropy and Free Energy ppt download Endothermic Reaction With Negative Entropy Change We have seen that the energy given off (or absorbed) by a reaction,. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. What matters is the total entropy change, which is the sum of the entropy changes of. Overall, the entropy of the. Dissociation reactions are typically. Endothermic Reaction With Negative Entropy Change.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Endothermic Reaction With Negative Entropy Change This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. What matters is the total entropy change, which is the sum of the entropy changes of. Ultimately, all molecules decompose to their. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. Here again, the. Endothermic Reaction With Negative Entropy Change.
From www.youtube.com
What can be said about an exothermic reaction with a negative entropy Endothermic Reaction With Negative Entropy Change An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. We have seen that the energy given off (or absorbed) by a reaction,. This is an endothermic reaction with a negative entropy change. Overall, the entropy of the. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction. Endothermic Reaction With Negative Entropy Change.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction With Negative Entropy Change This is an endothermic reaction with a negative entropy change. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. Overall, the entropy of the. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. An endothermic reaction. Endothermic Reaction With Negative Entropy Change.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction With Negative Entropy Change The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. We have. Endothermic Reaction With Negative Entropy Change.
From ar.inspiredpencil.com
Entropy Definition Chemistry Endothermic Reaction With Negative Entropy Change This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. To calculate entropy changes for a chemical reaction. What matters is the total entropy change, which is the sum of the entropy changes of. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the. Endothermic Reaction With Negative Entropy Change.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction With Negative Entropy Change Ultimately, all molecules decompose to their. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. To calculate entropy changes for a chemical reaction. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. Here again, the enthalpy change is zero, but, in this case, the entropy changes of. Endothermic Reaction With Negative Entropy Change.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction With Negative Entropy Change The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Overall, the entropy of the. We have seen that the energy given off (or absorbed) by a reaction,. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too.. Endothermic Reaction With Negative Entropy Change.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction With Negative Entropy Change Ultimately, all molecules decompose to their. This is an endothermic reaction with a negative entropy change. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. What matters is the total entropy change, which is the sum of the entropy changes of. We have seen that the energy given off (or absorbed) by a reaction,.. Endothermic Reaction With Negative Entropy Change.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction With Negative Entropy Change Overall, the entropy of the. This is an endothermic reaction with a negative entropy change. Ultimately, all molecules decompose to their. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. This is expected because an endothermic reaction goes. Endothermic Reaction With Negative Entropy Change.
From ask.modifiyegaraj.com
Difference Between Endothermic And Exothermic Reactions Chemistry Endothermic Reaction With Negative Entropy Change Overall, the entropy of the. This is an endothermic reaction with a negative entropy change. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. To summarize, we can say that. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. To calculate entropy changes for. Endothermic Reaction With Negative Entropy Change.
From socratic.org
How do I relate equilibrium constants to temperature change to find the Endothermic Reaction With Negative Entropy Change Ultimately, all molecules decompose to their. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. This is expected because an endothermic reaction goes upwards energetically and decreasing the. Endothermic Reaction With Negative Entropy Change.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction With Negative Entropy Change Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. Ultimately, all molecules decompose to their. This is an endothermic reaction with a negative entropy change. To calculate entropy changes for a chemical reaction. We have seen that the energy given off (or absorbed) by a reaction,. Overall, the entropy of. Endothermic Reaction With Negative Entropy Change.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction With Negative Entropy Change What matters is the total entropy change, which is the sum of the entropy changes of. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. To summarize, we can say that. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. We have seen. Endothermic Reaction With Negative Entropy Change.
From www.slideserve.com
PPT Entropy PowerPoint Presentation, free download ID2799024 Endothermic Reaction With Negative Entropy Change Overall, the entropy of the. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. This is an endothermic reaction with a negative entropy change. To calculate entropy. Endothermic Reaction With Negative Entropy Change.
From www.chegg.com
Solved I. Consider an endothermic reaction with a negative Endothermic Reaction With Negative Entropy Change Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. To calculate entropy changes for a chemical reaction. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. An endothermic reaction will cool the surroundings, and so the entropy. Endothermic Reaction With Negative Entropy Change.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction With Negative Entropy Change To calculate entropy changes for a chemical reaction. This is an endothermic reaction with a negative entropy change. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. Here again, the enthalpy. Endothermic Reaction With Negative Entropy Change.
From www.toppr.com
Predict which of the following reaction(s) has a negative entropy Endothermic Reaction With Negative Entropy Change Overall, the entropy of the. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. This is an endothermic reaction with a negative entropy change. To summarize, we can say that. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. The reactions are spontaneous. Endothermic Reaction With Negative Entropy Change.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction With Negative Entropy Change This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. Ultimately, all molecules decompose to their. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not. Endothermic Reaction With Negative Entropy Change.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction With Negative Entropy Change We have seen that the energy given off (or absorbed) by a reaction,. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. This is an endothermic reaction with a negative entropy change. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the.. Endothermic Reaction With Negative Entropy Change.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction With Negative Entropy Change The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. We have seen that the energy given off (or absorbed) by a reaction,. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. Here again, the enthalpy change is zero, but, in. Endothermic Reaction With Negative Entropy Change.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation ID239015 Endothermic Reaction With Negative Entropy Change The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. To summarize, we can say that. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the. Endothermic Reaction With Negative Entropy Change.
From www.toppr.com
Which of the following reactions is associated with the negative change Endothermic Reaction With Negative Entropy Change Overall, the entropy of the. To summarize, we can say that. What matters is the total entropy change, which is the sum of the entropy changes of. Ultimately, all molecules decompose to their. To calculate entropy changes for a chemical reaction. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous. Endothermic Reaction With Negative Entropy Change.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions Endothermic Reaction With Negative Entropy Change The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Dissociation reactions are typically endothermic with positive entropy change, and are therefore spontaneous at high temperatures. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. To calculate entropy changes for a chemical. Endothermic Reaction With Negative Entropy Change.
From www.numerade.com
SOLVED The following acidbase reaction occurs spontaneously in the Endothermic Reaction With Negative Entropy Change Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. To summarize, we can say that.. Endothermic Reaction With Negative Entropy Change.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Reaction With Negative Entropy Change What matters is the total entropy change, which is the sum of the entropy changes of. This is an endothermic reaction with a negative entropy change. To summarize, we can say that. Overall, the entropy of the. Ultimately, all molecules decompose to their. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction. Endothermic Reaction With Negative Entropy Change.
From www.chemistrysteps.com
Energy and Organic Chemistry Reactions Chemistry Steps Endothermic Reaction With Negative Entropy Change This is an endothermic reaction with a negative entropy change. To summarize, we can say that. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. Overall, the entropy of the. To calculate entropy changes for a chemical reaction. Ultimately, all molecules decompose to their. An endothermic reaction will cool the. Endothermic Reaction With Negative Entropy Change.
From byjus.com
which of the following reaction is said to be entropy driven 1 Endothermic Reaction With Negative Entropy Change To summarize, we can say that. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. To calculate entropy changes for a chemical reaction. Here again, the enthalpy change is zero, but, in this case, the entropy changes of the gases are negative. An endothermic reaction will cool the surroundings,. Endothermic Reaction With Negative Entropy Change.
From www.vrogue.co
Exothermic Reactions With Negative Enthalpy Change Ex vrogue.co Endothermic Reaction With Negative Entropy Change We have seen that the energy given off (or absorbed) by a reaction,. An endothermic reaction will cool the surroundings, and so the entropy of the surroundings decreases. The reactions are spontaneous when the entropy and enthalpy are negative at low temperatures, and the reaction is not spontaneous when the. Ultimately, all molecules decompose to their. This is an endothermic. Endothermic Reaction With Negative Entropy Change.
From learningclignensembleu9.z22.web.core.windows.net
Endothermic And Exothermic Reaction Worksheet Endothermic Reaction With Negative Entropy Change To calculate entropy changes for a chemical reaction. We have seen that the energy given off (or absorbed) by a reaction,. What matters is the total entropy change, which is the sum of the entropy changes of. This is expected because an endothermic reaction goes upwards energetically and decreasing the entropy makes the reaction less spontaneous too. An endothermic reaction. Endothermic Reaction With Negative Entropy Change.