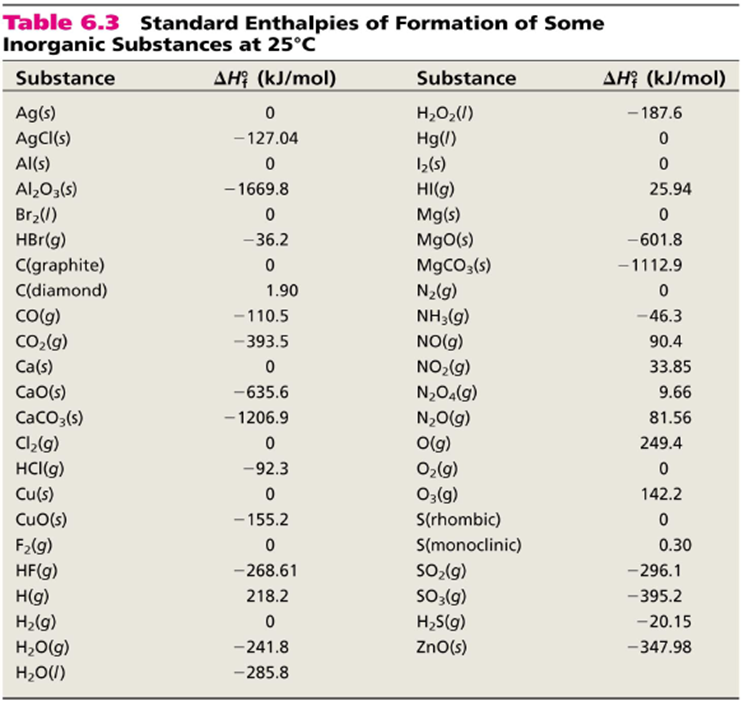
Standard Heat Of Formation Chart . The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
from rayb78.github.io
These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form.
Heat Of Formation Chart
Standard Heat Of Formation Chart These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+.
From kaden-chapter.blogspot.com
Standard Enthalpy Of Formation Table Pdf 30+ Pages Summary [1.8mb] Latest Update Kaden Books Standard Heat Of Formation Chart Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Heat Of Formation Chart.
From www.researchgate.net
Heat of formation and enthalpy data for slag compounds Enthalpy of... Download Table Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Standard Heat Of Formation Chart.
From chartdata.web.app
Standard Enthalpy Of Formation Chart Standard Heat Of Formation Chart The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. This table lists the standard enthalpies (δh°), the free energies (δg°). Standard Heat Of Formation Chart.
From www.scribd.com
Standard Heats of Formation, Hess's Law, Standard Enthalpy of Formation Table PDF Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol). Standard Heat Of Formation Chart.
From www.slideserve.com
PPT Energy Transformations PowerPoint Presentation, free download ID7001222 Standard Heat Of Formation Chart These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one. Standard Heat Of Formation Chart.
From wisataparatravell.blogspot.com
Standard Heats Of Formation Standard Enthalpies Of Formation And The Enthalpy Of The Reaction Standard Heat Of Formation Chart The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h. Standard Heat Of Formation Chart.
From www.semanticscholar.org
[PDF] Largescale calculations of gas phase thermochemistry Enthalpy of formation, standard Standard Heat Of Formation Chart These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Of Formation Chart.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Of Formation Chart.
From www.studocu.com
Heat of formation table STANDARD HEATS OF FORMATION FOR SELECTED SUBSTANCES Al 2 O 3 Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. The standard enthalpy change of formation is the sum of the heats of formation of the. Standard Heat Of Formation Chart.
From jamesherbert.z13.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Chart The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Heat Of Formation Chart.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Chart This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed. Standard Heat Of Formation Chart.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Thermochemistry Unit Standard Heat Of Formation Chart Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Heat Of Formation Chart.
From answerzonecarter.z13.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Chart The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Standard Heat Of Formation Chart.
From mavink.com
Standard Enthalpy Chart Standard Heat Of Formation Chart The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and. Standard Heat Of Formation Chart.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Chart Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The table below shows the standard enthalpy of formation, the standard gibbs free energy of. Standard Heat Of Formation Chart.
From mavink.com
Standard Enthalpy Chart Standard Heat Of Formation Chart Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. The standard enthalpy change of formation is the sum of the heats of formation of the. Standard Heat Of Formation Chart.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol ) ( ) g 0 223 aq 56 aq 182 s Standard Heat Of Formation Chart Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. The standard enthalpy change of formation is the sum of the heats of formation of the. Standard Heat Of Formation Chart.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation* for Various Compounds Standard Heat Of Formation Chart This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. The standard enthalpy change of formation is the sum of the heats of formation of the. Standard Heat Of Formation Chart.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Heat Of Formation Chart Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Heat Of Formation Chart.
From www.researchgate.net
The calculated M index and the experimental standard enthalpy of... Download Table Standard Heat Of Formation Chart Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. This table lists the standard enthalpies (δh°), the free energies (δg°). Standard Heat Of Formation Chart.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs Standard Heat Of Formation Chart This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. The standard enthalpy of formation is a measure of the energy released or consumed when one. Standard Heat Of Formation Chart.
From www.slideshare.net
Heat of formation by reactions Standard Heat Of Formation Chart Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. These tables include heat of formation data gathered from a variety of sources, including the primary and. Standard Heat Of Formation Chart.
From www.semanticscholar.org
Table I from Largescale calculations of gas phase thermochemistry Enthalpy of formation Standard Heat Of Formation Chart Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds. Standard Heat Of Formation Chart.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Heat Of Formation Chart Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Of Formation Chart.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. These tables include heat of formation data gathered from a variety of sources, including the primary. Standard Heat Of Formation Chart.
From learningschoolandy.z21.web.core.windows.net
Heat Of Formation List Standard Heat Of Formation Chart These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one. Standard Heat Of Formation Chart.
From chartdata.web.app
Standard Enthalpy Of Formation Chart Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f). Standard Heat Of Formation Chart.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Heat Of Formation Chart 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. These tables include heat of formation data gathered from a variety of sources,. Standard Heat Of Formation Chart.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Heat Of Formation Chart Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when. Standard Heat Of Formation Chart.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Of Formation Chart 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. These tables include heat of formation data gathered from a variety of sources, including the primary. Standard Heat Of Formation Chart.
From classhirsch.z21.web.core.windows.net
Standard Enthalpy Of Formation Chart Standard Heat Of Formation Chart The table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar heat. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol). Standard Heat Of Formation Chart.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Chart The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation is the sum of the heats of. Standard Heat Of Formation Chart.
From mungfali.com
Enthalpies Of Formation Chart Standard Heat Of Formation Chart Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Of Formation Chart.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Heat Of Formation Chart The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. These tables include heat of formation data gathered from a variety of sources, including the primary and secondary literature, as well as the. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Heat Of Formation Chart.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Of Formation Chart The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. These tables include heat of formation data gathered from. Standard Heat Of Formation Chart.