What Are The Buffer System . A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is buffer in chemistry? A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. It is able to neutralize small amounts of added acid or base, thus. Proteins assist with intracellular ph.
from www.youtube.com
It is able to neutralize small amounts of added acid or base, thus. Proteins assist with intracellular ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is buffer in chemistry? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
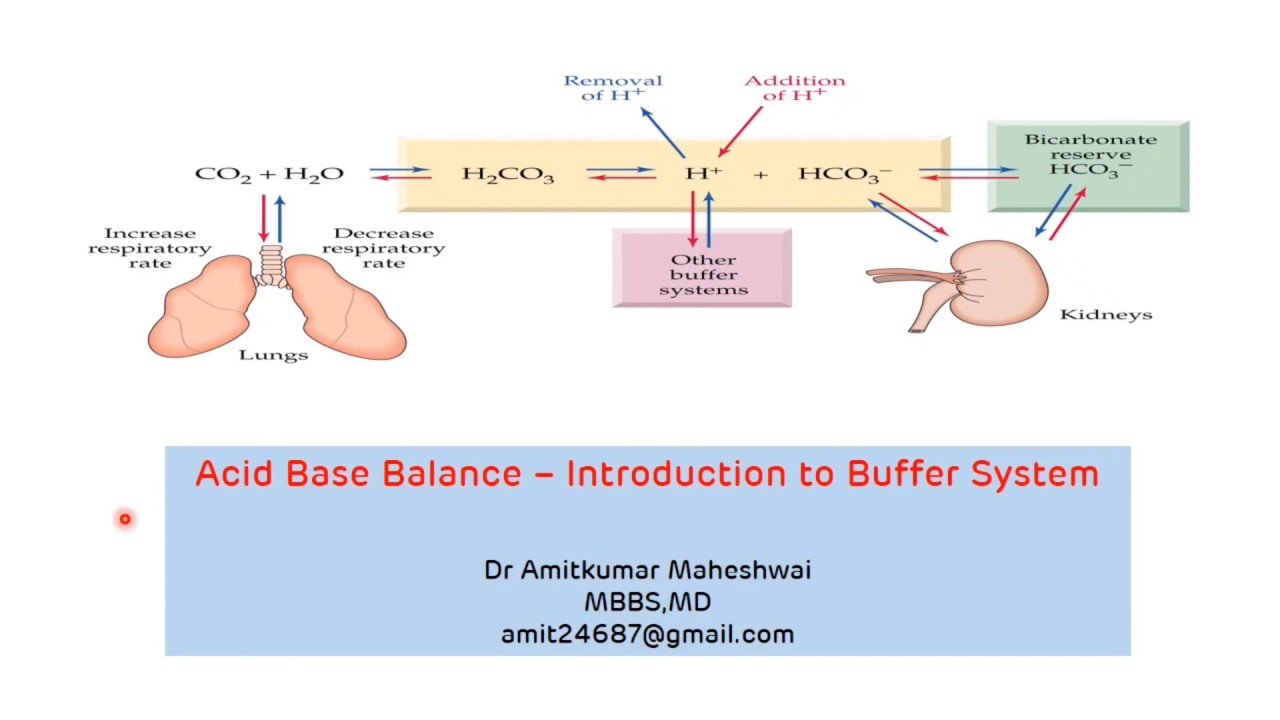
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in
What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. Proteins assist with intracellular ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The phosphate buffer system, while present globally, is important for the regulation of urine ph. What is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Are The Buffer System A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The phosphate buffer system, while present globally, is important for the. What Are The Buffer System.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation ID297131 What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. What is buffer in. What Are The Buffer System.
From www.slideshare.net
Buffer system What Are The Buffer System A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A solution whose ph is not altered to any great extent by the addition of small quantities of. What Are The Buffer System.
From www.slideshare.net
Buffer system What Are The Buffer System What is buffer in chemistry? A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Proteins assist with intracellular ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The phosphate buffer system, while present globally, is important. What Are The Buffer System.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free download ID4769309 What Are The Buffer System A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. What Are The Buffer System.
From www.slideserve.com
PPT Acid Base Balance PowerPoint Presentation, free download ID2413658 What Are The Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A mixture. What Are The Buffer System.
From animalia-life.club
Phosphate Buffer System Equation What Are The Buffer System A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small. What Are The Buffer System.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Presentation ID2687569 What Are The Buffer System What is buffer in chemistry? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even. What Are The Buffer System.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Are The Buffer System A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. What is buffer in chemistry? A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that. What Are The Buffer System.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download ID9427787 What Are The Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is buffer in chemistry? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or. What Are The Buffer System.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Are The Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A solution whose. What Are The Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching chemistry, Medical school What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What is buffer in chemistry? A variety of buffering systems permits blood and other bodily fluids to. What Are The Buffer System.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation ID297131 What Are The Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph. It is able to neutralize small amounts of added acid or base, thus. A solution whose ph is. What Are The Buffer System.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. It is able to neutralize small amounts of added acid or base, thus. What. What Are The Buffer System.
From www.slideshare.net
Buffer system What Are The Buffer System A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Proteins assist with intracellular ph. It is able to neutralize small amounts of added acid or base, thus. The phosphate buffer system, while present globally, is important for the regulation of urine ph.. What Are The Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Are The Buffer System A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. What is buffer in chemistry? A variety of buffering systems permits. What Are The Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Are The Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. It is. What Are The Buffer System.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Proteins assist with intracellular ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A variety of buffering systems permits blood and other. What Are The Buffer System.
From ar.inspiredpencil.com
Renal Buffer System What Are The Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid. What Are The Buffer System.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in What Are The Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. Proteins. What Are The Buffer System.
From operaresidences.com.au
Describe the role of the phosphate buffer system? Opera Residences What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The phosphate buffer system, while present globally, is important for the regulation of urine ph. It is able to neutralize small amounts of added acid or base, thus. A solution whose ph is not altered to any great extent by the. What Are The Buffer System.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. Proteins assist with intracellular ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base. What Are The Buffer System.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and bicarbonate buffer system YouTube What Are The Buffer System Proteins assist with intracellular ph. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. What is buffer in chemistry? A solution whose ph is not altered to. What Are The Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Are The Buffer System The phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A mixture of a weak acid and its conjugate base (or a mixture of a weak. What Are The Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Are The Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Proteins assist with intracellular ph. The phosphate buffer system, while present globally, is important for the. What Are The Buffer System.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Alkalosis What Are The Buffer System A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What is buffer in chemistry? Proteins assist with intracellular ph. The phosphate buffer system, while present globally, is important for the regulation of urine ph. It is able to neutralize small amounts of added acid or. What Are The Buffer System.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood plasma components What Are The Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. What is buffer in chemistry? Proteins assist with intracellular ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A. What Are The Buffer System.
From www.slideshare.net
Buffer system What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Proteins assist with intracellular ph. What is buffer in chemistry? The phosphate buffer system, while present globally,. What Are The Buffer System.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online presentation What Are The Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A solution whose ph is not altered to any great extent. What Are The Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Are The Buffer System Proteins assist with intracellular ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A. What Are The Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Are The Buffer System Proteins assist with intracellular ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that maintains the stability. What Are The Buffer System.
From study.com
Buffer System in Chemistry Definition, Function & Examples Lesson What Are The Buffer System What is buffer in chemistry? Proteins assist with intracellular ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a. What Are The Buffer System.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner What Are The Buffer System What is buffer in chemistry? A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. The. What Are The Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Are The Buffer System It is able to neutralize small amounts of added acid or base, thus. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of. A. What Are The Buffer System.
From www.slideshare.net
Blood buffer system What Are The Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. The phosphate buffer system, while present globally, is important for the regulation of urine ph. A buffer is a solution that maintains the stability of a system’s ph. What Are The Buffer System.