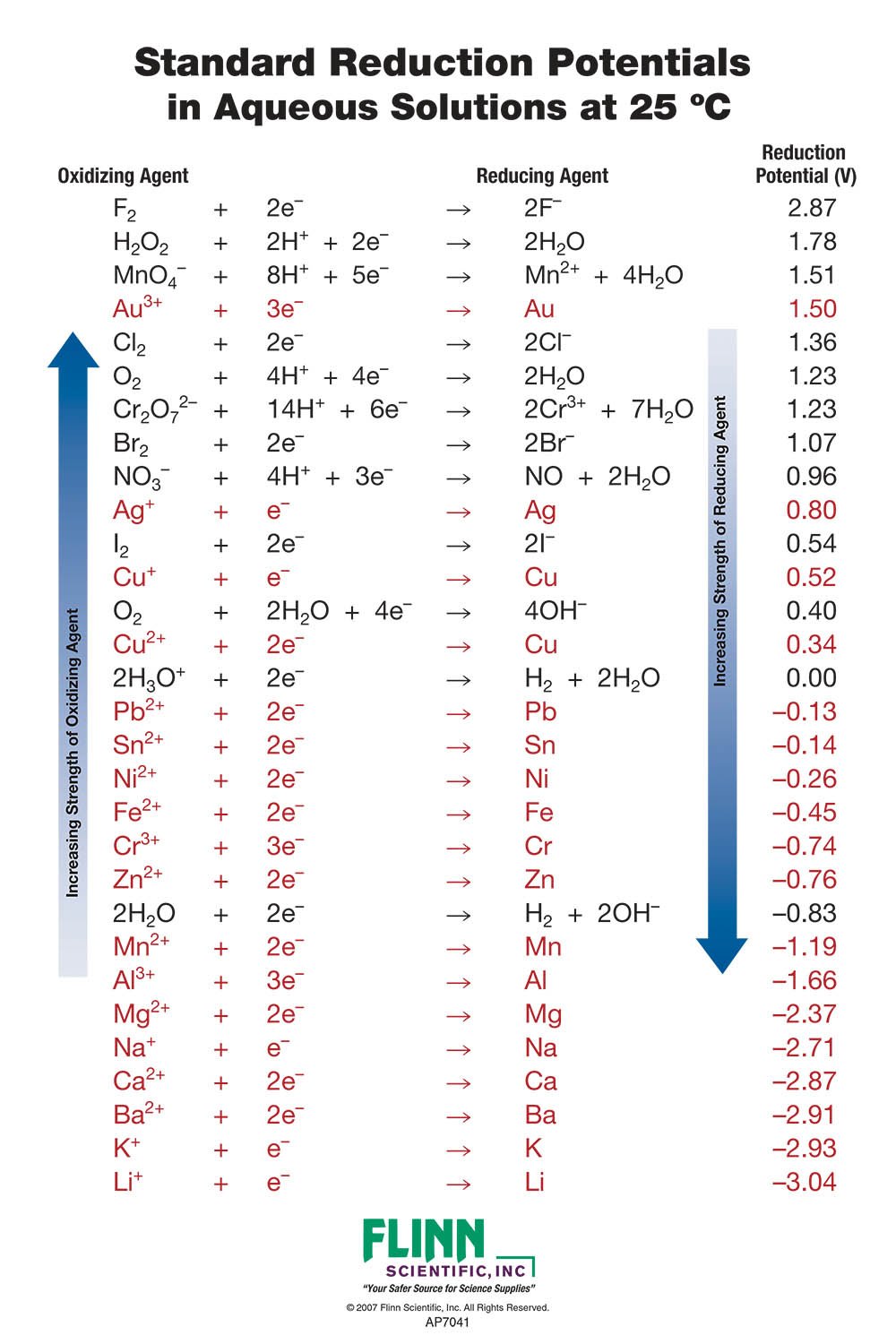
Standard Reduction Potential Of Galvanic Cell . The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. · be able to predict the cell potential for a galvanic cell using a reference. A galvanic cell can be used to determine the standard reduction potential of cu2 +. To find the difference of the two half cells, the following equation is used: By the end of this lesson, students will: The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. Using the she as a reference, other standard reduction. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of.
from www.flinnsci.ca
To find the difference of the two half cells, the following equation is used: A galvanic cell can be used to determine the standard reduction potential of cu2 +. · be able to predict the cell potential for a galvanic cell using a reference. Using the she as a reference, other standard reduction. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. By the end of this lesson, students will: The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell.
Standard Reduction Potential Charts for Chemistry
Standard Reduction Potential Of Galvanic Cell · be able to predict the cell potential for a galvanic cell using a reference. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. By the end of this lesson, students will: To find the difference of the two half cells, the following equation is used: A galvanic cell can be used to determine the standard reduction potential of cu2 +. · be able to predict the cell potential for a galvanic cell using a reference. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Using the she as a reference, other standard reduction.
From www.nagwa.com
Question Video Calculating the Standard Reduction Potential of an Standard Reduction Potential Of Galvanic Cell To find the difference of the two half cells, the following equation is used: By the end of this lesson, students will: A galvanic cell can be used to determine the standard reduction potential of cu2 +. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in. Standard Reduction Potential Of Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Standard Reduction Potential Of Galvanic Cell A galvanic cell can be used to determine the standard reduction potential of cu2 +. · be able to predict the cell potential for a galvanic cell using a reference. Using the she as a reference, other standard reduction. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. By the. Standard Reduction Potential Of Galvanic Cell.
From www.slideserve.com
PPT 19.2 Galvanic Cells 19.3 Standard Reduction Potentials 19.4 Standard Reduction Potential Of Galvanic Cell By the end of this lesson, students will: To find the difference of the two half cells, the following equation is used: The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. A galvanic cell can be constructed from a zinc electrode immersed in a solution of. Standard Reduction Potential Of Galvanic Cell.
From www.slideserve.com
PPT 19.2 Galvanic Cells 19.3 Standard Reduction Potentials 19.4 Standard Reduction Potential Of Galvanic Cell Using the she as a reference, other standard reduction. By the end of this lesson, students will: A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and. Standard Reduction Potential Of Galvanic Cell.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Of Galvanic Cell The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. By the end of this lesson, students will: Using the she as a reference, other standard reduction. To find the difference of the two half cells, the following equation is used: A galvanic cell can be used. Standard Reduction Potential Of Galvanic Cell.
From www.bartleby.com
Answered A chemist designs a galvanic cell that… bartleby Standard Reduction Potential Of Galvanic Cell By the end of this lesson, students will: A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. Using the she as a reference, other standard reduction. A galvanic cell can be used to determine the standard reduction potential of cu2 +. To find. Standard Reduction Potential Of Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Standard Reduction Potential Of Galvanic Cell By the end of this lesson, students will: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. A galvanic cell can be constructed from a zinc electrode. Standard Reduction Potential Of Galvanic Cell.
From www.coursehero.com
[Solved] D) Galvanic Cell Use the standard reduction potentials to Standard Reduction Potential Of Galvanic Cell The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. By the end of this lesson, students will: A galvanic cell can be used to. Standard Reduction Potential Of Galvanic Cell.
From www.studypool.com
SOLUTION To determine the standard reduction potential galvanic cell Standard Reduction Potential Of Galvanic Cell A galvanic cell can be used to determine the standard reduction potential of cu2 +. By the end of this lesson, students will: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following equation is used: · be able to. Standard Reduction Potential Of Galvanic Cell.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Of Galvanic Cell To find the difference of the two half cells, the following equation is used: By the end of this lesson, students will: A galvanic cell can be used to determine the standard reduction potential of cu2 +. Using the she as a reference, other standard reduction. The voltmeter shows that the standard cell potential of a galvanic cell consisting of. Standard Reduction Potential Of Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Standard Reduction Potential Of Galvanic Cell · be able to predict the cell potential for a galvanic cell using a reference. Using the she as a reference, other standard reduction. By the end of this lesson, students will: To find the difference of the two half cells, the following equation is used: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms. Standard Reduction Potential Of Galvanic Cell.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Standard Reduction Potential Of Galvanic Cell · be able to predict the cell potential for a galvanic cell using a reference. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell.. Standard Reduction Potential Of Galvanic Cell.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Of Galvanic Cell The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. By the end of this lesson, students will: The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. · be able to predict the cell potential for a. Standard Reduction Potential Of Galvanic Cell.
From www.chegg.com
Solved Galvanic Cells Using tabulated standard reduction Standard Reduction Potential Of Galvanic Cell The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. A galvanic cell can be used to determine the standard reduction potential of cu2 +. By the end of this lesson, students will: Using the she as a reference, other standard reduction. A galvanic cell can be constructed from a zinc. Standard Reduction Potential Of Galvanic Cell.
From www.numerade.com
SOLVED A certain halfreaction has standard reduction potential E" 0. Standard Reduction Potential Of Galvanic Cell Using the she as a reference, other standard reduction. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. A galvanic cell can be used to determine the standard reduction potential of cu2 +. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate. Standard Reduction Potential Of Galvanic Cell.
From www.studypool.com
SOLUTION Lecture05 standard and nonstandard reduction potential Standard Reduction Potential Of Galvanic Cell A galvanic cell can be used to determine the standard reduction potential of cu2 +. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. · be able to predict the cell potential for a galvanic cell using a reference. The voltmeter shows that the standard cell potential of a galvanic. Standard Reduction Potential Of Galvanic Cell.
From www.slideserve.com
PPT 19.2 Galvanic Cells 19.3 Standard Reduction Potentials 19.4 Standard Reduction Potential Of Galvanic Cell The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and. Standard Reduction Potential Of Galvanic Cell.
From joiujlkil.blob.core.windows.net
Standard Cell Potential Galvanic at Rhonda Beecher blog Standard Reduction Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. To find the difference of the two half cells, the following equation is used: · be able to predict the cell potential for a galvanic cell using a reference. The standard cell potential (\(e^o_{cell}\)). Standard Reduction Potential Of Galvanic Cell.
From www.chegg.com
The standard potential for the following galvanic Standard Reduction Potential Of Galvanic Cell To find the difference of the two half cells, the following equation is used: · be able to predict the cell potential for a galvanic cell using a reference. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The standard cell potential (\(e^o_{cell}\)). Standard Reduction Potential Of Galvanic Cell.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Reduction Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. Using the she as a reference, other standard reduction. By the end of this lesson, students will: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of. Standard Reduction Potential Of Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Standard Reduction Potential Of Galvanic Cell By the end of this lesson, students will: A galvanic cell can be used to determine the standard reduction potential of cu2 +. A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. To find the difference of the two half cells, the following. Standard Reduction Potential Of Galvanic Cell.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Reduction Potential Of Galvanic Cell A galvanic cell can be used to determine the standard reduction potential of cu2 +. · be able to predict the cell potential for a galvanic cell using a reference. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. To find the difference of the two. Standard Reduction Potential Of Galvanic Cell.
From www.chegg.com
Solved A galvanic cell using Au+/ Au and Ni2+/Ni was set up Standard Reduction Potential Of Galvanic Cell The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Using the she as a reference, other standard reduction. By the end of this lesson, students will: · be able to predict the cell potential for a galvanic cell using a reference. A galvanic cell can be constructed from a zinc. Standard Reduction Potential Of Galvanic Cell.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Galvanic Cells PowerPoint Standard Reduction Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. To find the difference of the two half cells, the following equation is used: Using the she as a reference, other standard reduction. The standard cell potential (\(e^o_{cell}\)) is the difference of the two. Standard Reduction Potential Of Galvanic Cell.
From www.studypool.com
SOLUTION Lecture05 standard and nonstandard reduction potential Standard Reduction Potential Of Galvanic Cell · be able to predict the cell potential for a galvanic cell using a reference. A galvanic cell can be used to determine the standard reduction potential of cu2 +. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. The standard cell potential (\(e^o_{cell}\)) is the. Standard Reduction Potential Of Galvanic Cell.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Standard Reduction Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following equation is used: The. Standard Reduction Potential Of Galvanic Cell.
From www.chegg.com
Solved The cell potential for the standard galvanic cell Standard Reduction Potential Of Galvanic Cell To find the difference of the two half cells, the following equation is used: A galvanic cell can be used to determine the standard reduction potential of cu2 +. By the end of this lesson, students will: Using the she as a reference, other standard reduction. · be able to predict the cell potential for a galvanic cell using a. Standard Reduction Potential Of Galvanic Cell.
From slideplayer.com
Voltaic (Galvanic)Cells ppt download Standard Reduction Potential Of Galvanic Cell Using the she as a reference, other standard reduction. A galvanic cell can be used to determine the standard reduction potential of cu2 +. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. By the end of this lesson, students will: To find the difference of. Standard Reduction Potential Of Galvanic Cell.
From chemcollective.org
Electrochemistry Galvanic Cells and the Nernst Equation Standard Reduction Potential Of Galvanic Cell A galvanic cell can be constructed from a zinc electrode immersed in a solution of zinc sulfate and an iron electrode immersed in a solution of. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. · be able to predict the cell potential for a galvanic. Standard Reduction Potential Of Galvanic Cell.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Reduction Potential Of Galvanic Cell By the end of this lesson, students will: Using the she as a reference, other standard reduction. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. · be able to predict the cell potential for a galvanic cell using a reference. To find the difference of the two half cells,. Standard Reduction Potential Of Galvanic Cell.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts Standard Reduction Potential Of Galvanic Cell · be able to predict the cell potential for a galvanic cell using a reference. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. To find the difference of the two half cells, the following equation is used: The standard cell potential (\(e^o_{cell}\)) is the difference. Standard Reduction Potential Of Galvanic Cell.
From www.studypool.com
SOLUTION Standard and Nonstandard Reduction Potential Calculations for Standard Reduction Potential Of Galvanic Cell To find the difference of the two half cells, the following equation is used: Using the she as a reference, other standard reduction. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a. Standard Reduction Potential Of Galvanic Cell.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Standard Reduction Potential Of Galvanic Cell The voltmeter shows that the standard cell potential of a galvanic cell consisting of a she and a zn/zn 2 + couple is. A galvanic cell can be used to determine the standard reduction potential of cu2 +. To find the difference of the two half cells, the following equation is used: By the end of this lesson, students will:. Standard Reduction Potential Of Galvanic Cell.
From chem.libretexts.org
19.5 Standard Electrochemical Potentials Chemistry LibreTexts Standard Reduction Potential Of Galvanic Cell · be able to predict the cell potential for a galvanic cell using a reference. Using the she as a reference, other standard reduction. A galvanic cell can be used to determine the standard reduction potential of cu2 +. To find the difference of the two half cells, the following equation is used: A galvanic cell can be constructed from. Standard Reduction Potential Of Galvanic Cell.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Reduction Potential Of Galvanic Cell By the end of this lesson, students will: A galvanic cell can be used to determine the standard reduction potential of cu2 +. Using the she as a reference, other standard reduction. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells,. Standard Reduction Potential Of Galvanic Cell.