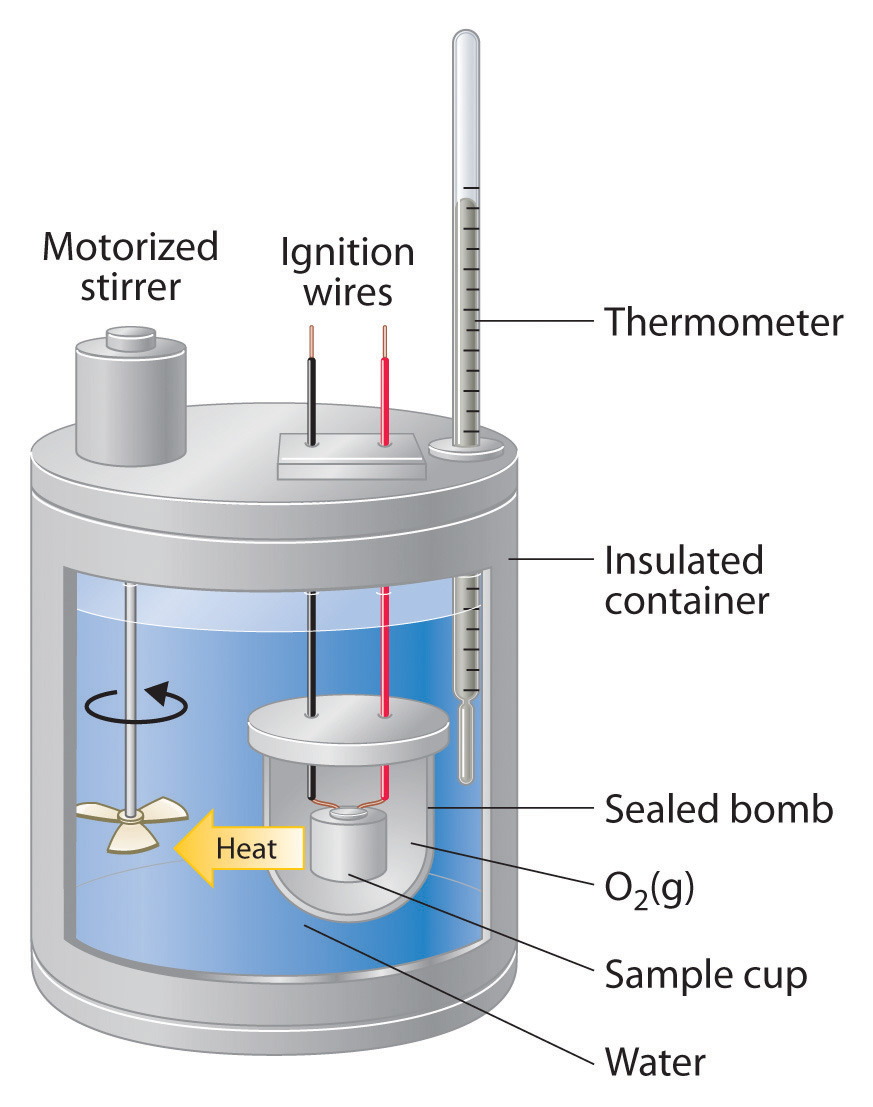
Calorimetry Experiments . One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to determine heat flow during a chemical or physical change. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. A doubled styrofoam cup fitted. We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry.
from
We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to determine heat flow during a chemical or physical change. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. A doubled styrofoam cup fitted. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation.
Calorimetry Experiments One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. a calorimeter is a device used to determine heat flow during a chemical or physical change. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A doubled styrofoam cup fitted. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Experiments A doubled styrofoam cup fitted. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. One technique we can use to measure the amount of heat involved. Calorimetry Experiments.
From
Calorimetry Experiments a calorimeter is a device used to determine heat flow during a chemical or physical change. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. . Calorimetry Experiments.
From
Calorimetry Experiments Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into. Calorimetry Experiments.
From
Calorimetry Experiments calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and. Calorimetry Experiments.
From
Calorimetry Experiments calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A doubled styrofoam cup fitted. the amount of heat energy released by a chemical reaction can be measured. Calorimetry Experiments.
From
Calorimetry Experiments a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We use capital \(c\) to represent the heat capacitance of an object, so for the. Calorimetry Experiments.
From www.youtube.com
Experiment 3 Calorimetry YouTube Calorimetry Experiments the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. a calorimeter is a device used to determine heat flow during a chemical or physical change. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are.. Calorimetry Experiments.
From
Calorimetry Experiments We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. One technique we can use to measure the amount of heat involved in a chemical or physical process. Calorimetry Experiments.
From
Calorimetry Experiments One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A doubled styrofoam cup fitted. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. calorimetry allows for the measurement of the amount of energy transferred. Calorimetry Experiments.
From
Calorimetry Experiments calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat. Calorimetry Experiments.
From
Calorimetry Experiments A doubled styrofoam cup fitted. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity. Calorimetry Experiments.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiments A doubled styrofoam cup fitted. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. We use capital \(c\) to represent the heat capacitance of an object, so. Calorimetry Experiments.
From
Calorimetry Experiments Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. a calorimeter is a device used to determine heat flow during a chemical or physical. Calorimetry Experiments.
From exonegtdm.blob.core.windows.net
A Level Chemistry Calorimetry Practical Aqa at Richelle Doty blog Calorimetry Experiments the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we. Calorimetry Experiments.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetry Experiments calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. We use capital \(c\) to represent the heat capacitance of an object, so for the. Calorimetry Experiments.
From exohkbfgq.blob.core.windows.net
Calorimeter For Experiment at Lillian Bordner blog Calorimetry Experiments a calorimeter is a device used to determine heat flow during a chemical or physical change. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. the amount. Calorimetry Experiments.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Experiments experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. a calorimeter is a device used to determine heat flow during a chemical or physical change. One technique we can use to measure the amount of heat involved in a chemical or physical. Calorimetry Experiments.
From
Calorimetry Experiments A doubled styrofoam cup fitted. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. a vessel that provides adequate heat lead (solid) 0.129 insulation from. Calorimetry Experiments.
From
Calorimetry Experiments We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. the amount of heat energy released by a chemical reaction can be. Calorimetry Experiments.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimetry Experiments Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter. Calorimetry Experiments.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Experiments A doubled styrofoam cup fitted. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water. Calorimetry Experiments.
From youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Experiments the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. A doubled styrofoam cup fitted. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. to make sure you get accurate results you need to calculate the calorimeter. Calorimetry Experiments.
From
Calorimetry Experiments calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving. Calorimetry Experiments.
From learninglibjohn88.z19.web.core.windows.net
Coffee Cup Calorimetry Experiment Calorimetry Experiments the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel. Calorimetry Experiments.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Experiments A doubled styrofoam cup fitted. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to determine heat flow during a chemical or physical change. We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter. Calorimetry Experiments.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimetry Experiments We use capital \(c\) to represent the heat capacitance of an object, so for the calorimeter constant we will use \(c_{cal}\). A doubled styrofoam cup fitted. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. to make sure you get accurate results you need to calculate the calorimeter. Calorimetry Experiments.
From
Calorimetry Experiments One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving. Calorimetry Experiments.
From www.thetechedvocate.org
How to Build a Calorimeter The Tech Edvocate Calorimetry Experiments a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. One technique we can use to measure the amount of heat. Calorimetry Experiments.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog Calorimetry Experiments a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel (solid) 0.443 temperature changes are. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. One technique we can use to measure the amount of heat involved in a chemical or. Calorimetry Experiments.
From users.highland.edu
Calorimetry Calorimetry Experiments the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a. Calorimetry Experiments.
From
Calorimetry Experiments experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. A doubled styrofoam cup fitted. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. to make sure you get accurate. Calorimetry Experiments.
From
Calorimetry Experiments to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. We use capital. Calorimetry Experiments.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Experiments A doubled styrofoam cup fitted. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated. We use capital \(c\) to represent the heat capacitance of an object, so for the. Calorimetry Experiments.
From saylordotorg.github.io
Calorimetry Calorimetry Experiments experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. We use capital \(c\) to represent the heat capacitance of an object, so for. Calorimetry Experiments.
From
Calorimetry Experiments the amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a vessel that provides adequate heat lead (solid) 0.129 insulation from its surroundings, and in which nickel. Calorimetry Experiments.