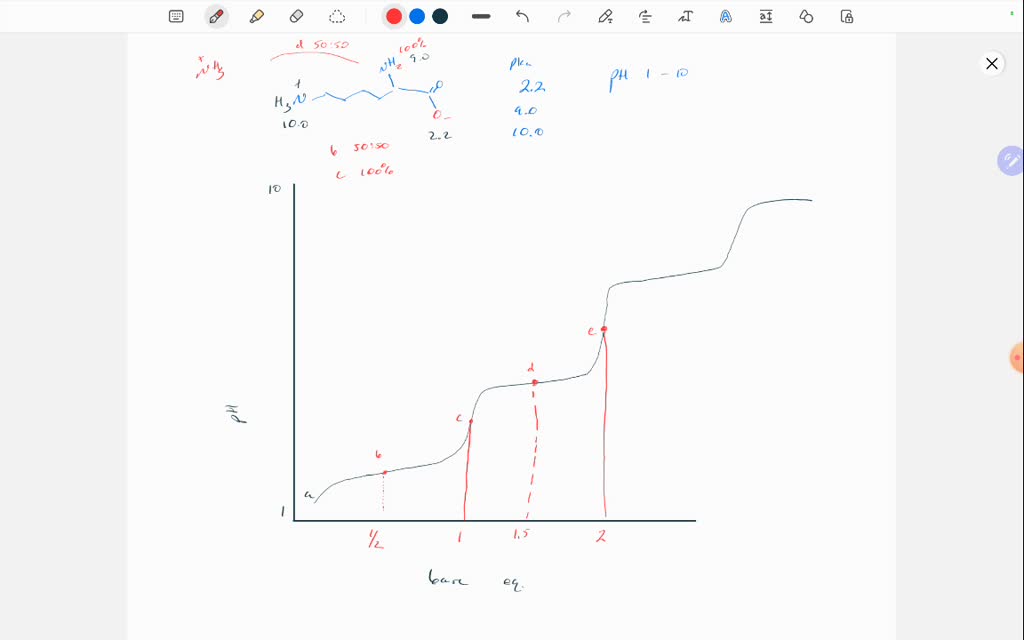
Titration Pka . to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. — the titration curve is a graphical representation of a titration in which the volume of.
from www.numerade.com
to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. — the titration curve is a graphical representation of a titration in which the volume of. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations.
SOLVED The pKa values for lysine are 2.2, 9.0 and 10.0. Draw a
Titration Pka — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — the titration curve is a graphical representation of a titration in which the volume of. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. the middle line represents the pka, while the two outer lines represent the end or start of the color changes.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Pka the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — the titration curve is a graphical representation of a titration in which the volume of. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. . Titration Pka.
From www.numerade.com
SOLVED Clearly draw the titration curve for the reaction of this amino Titration Pka The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. the middle line represents the pka, while the two outer lines represent the end. Titration Pka.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Pka to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. Pick a point on your titration curve prior to the equivalence point and record its ph,. Titration Pka.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Pka the middle line represents the pka, while the two outer lines represent the end or start of the color changes. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. — the titration curve is a graphical representation of a titration in which the. Titration Pka.
From www.transtutors.com
(Get Answer) Transcribed image text The graph shows the titration Titration Pka The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. the titration curves shown in figure 14.20 illustrate the choice of a suitable. Titration Pka.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Pka The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. — it consists in titrating a solution of the test compound with an acid or. Titration Pka.
From www.numerade.com
titration curve of aspartic acid is shown, determine the pl value for Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. to calculate the. Titration Pka.
From www.reddit.com
Enzyme Titration Curve pKa Identification chemhelp Titration Pka the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — the titration curve is a graphical representation of a titration in which the volume of. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. the. Titration Pka.
From www.youtube.com
Titration of Phosphoric Acid + pKa Values You Should Memorize MCAT Titration Pka the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. the middle line represents the pka, while the two outer lines represent the end or start of. Titration Pka.
From www.youtube.com
pKa by Potentiometric titration Dr. Sanjay Gaikwad YouTube Titration Pka the middle line represents the pka, while the two outer lines represent the end or start of the color changes. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. The peak and light blue highlights show the range in which the color changes will. Titration Pka.
From mavink.com
Pka Titration Curve Titration Pka The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. — the titration curve is a graphical representation of a titration in which the volume of. — it consists in titrating a solution of the test compound with an acid or a base of perfectly. Titration Pka.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration Pka Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the middle line represents the pka, while the two outer lines represent the end or start of. Titration Pka.
From www.researchgate.net
(A) NMR titration of the PKAthiophosphorylated form of ¹⁵Nlabeled Titration Pka Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. — the titration curve is a graphical representation of a titration in which the volume of. the middle line represents the pka, while the two outer lines represent the end or start of the. Titration Pka.
From www.researchgate.net
Titration curves of weak diacids with pK a1 = 4 and pK a2 = 5 or 5.3, C Titration Pka the middle line represents the pka, while the two outer lines represent the end or start of the color changes. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator. Titration Pka.
From sciencing.com
How to Calculate the PKA in Titration Sciencing Titration Pka the middle line represents the pka, while the two outer lines represent the end or start of the color changes. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known. Titration Pka.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. —. Titration Pka.
From www.reddit.com
in pH titration, how do you determine the (multiple) pKa points from Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. The peak and light blue highlights show the range in which the color changes will occur based on the amount. Titration Pka.
From www.youtube.com
Amino acid titration curve pH, pKa, pI concepts simplified YouTube Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,.. Titration Pka.
From general.chemistrysteps.com
pH and pKa Relationship Chemistry Steps Titration Pka to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — it consists in titrating a solution of the test compound with an acid or a base of. Titration Pka.
From www.numerade.com
SOLVED The pKa values for lysine are 2.2, 9.0 and 10.0. Draw a Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. — it consists in titrating a solution of the test compound with an acid or a base of. Titration Pka.
From www.researchgate.net
Titration curves of weak diacids with pK a1 = 4 and pK a2 = 5 or 5.3, C Titration Pka — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. — the titration curve is a graphical representation of a titration in which the volume of. The peak and light blue highlights show the range in which the color changes will occur based on the amount of. Titration Pka.
From www.youtube.com
Titration curves, pKa, pI, and Buffering regions YouTube Titration Pka the middle line represents the pka, while the two outer lines represent the end or start of the color changes. — the titration curve is a graphical representation of a titration in which the volume of. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,.. Titration Pka.
From www.chegg.com
Solved Below is the titration curve for histidine. The pKa Titration Pka to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. — the titration curve is a graphical representation of a titration in which the volume of. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical. Titration Pka.
From www.researchgate.net
(PDF) Methods for pKa Determination (I) Potentiometry Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. The peak and light blue highlights show the range in which the color changes will occur based on the amount of. Titration Pka.
From www.researchgate.net
Titration of PKA and PKG by PKI78 and PKI70 inhibitor protein Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the. Titration Pka.
From solvedlib.com
Given the following titration curve estimate the pKa … SolvedLib Titration Pka — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. The peak and light blue highlights show the range in which the color changes will. Titration Pka.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. — it consists in titrating a solution of the test compound with an acid or a base of perfectly. Titration Pka.
From solvedlib.com
Given the following titration curve estimate the pKa … SolvedLib Titration Pka The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. Pick a point on your titration curve prior to the equivalence point and record. Titration Pka.
From www.researchgate.net
Titration of PKA by three substrate competitive protein kinase Titration Pka the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the middle line represents the pka, while the two outer lines represent the end or start of the color changes. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate. Titration Pka.
From chemistry.stackexchange.com
Titration of CH3COONa with HCl and pKa determination from half Titration Pka The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. — it consists in titrating a solution of the test compound with an. Titration Pka.
From www.researchgate.net
Titration curve of BMAA. Titration shows two pK a values, primary amine Titration Pka — it consists in titrating a solution of the test compound with an acid or a base of perfectly known concentration,. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the middle line represents the pka, while the two outer lines represent the end or start of the color. Titration Pka.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Pka — the titration curve is a graphical representation of a titration in which the volume of. Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. The peak and light blue highlights show the range in which the color changes will occur based on the. Titration Pka.
From www.youtube.com
Titration Part 8 Read pKa YouTube Titration Pka the middle line represents the pka, while the two outer lines represent the end or start of the color changes. to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. — it consists in titrating a solution of the test compound with an acid or. Titration Pka.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Pka to calculate the pka of the solution, firstly, we will determine the equivalence point and then find the ph of the solution. The peak and light blue highlights show the range in which the color changes will occur based on the amount of titrant added. the titration curves shown in figure 14.20 illustrate the choice of a suitable. Titration Pka.
From www.vrogue.co
How To Draw Titration Curve Given Pka Youtube vrogue.co Titration Pka Pick a point on your titration curve prior to the equivalence point and record its ph, which is the vertical coordinate of the curve. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. — the titration curve is a graphical representation of a titration in which the volume of. . Titration Pka.