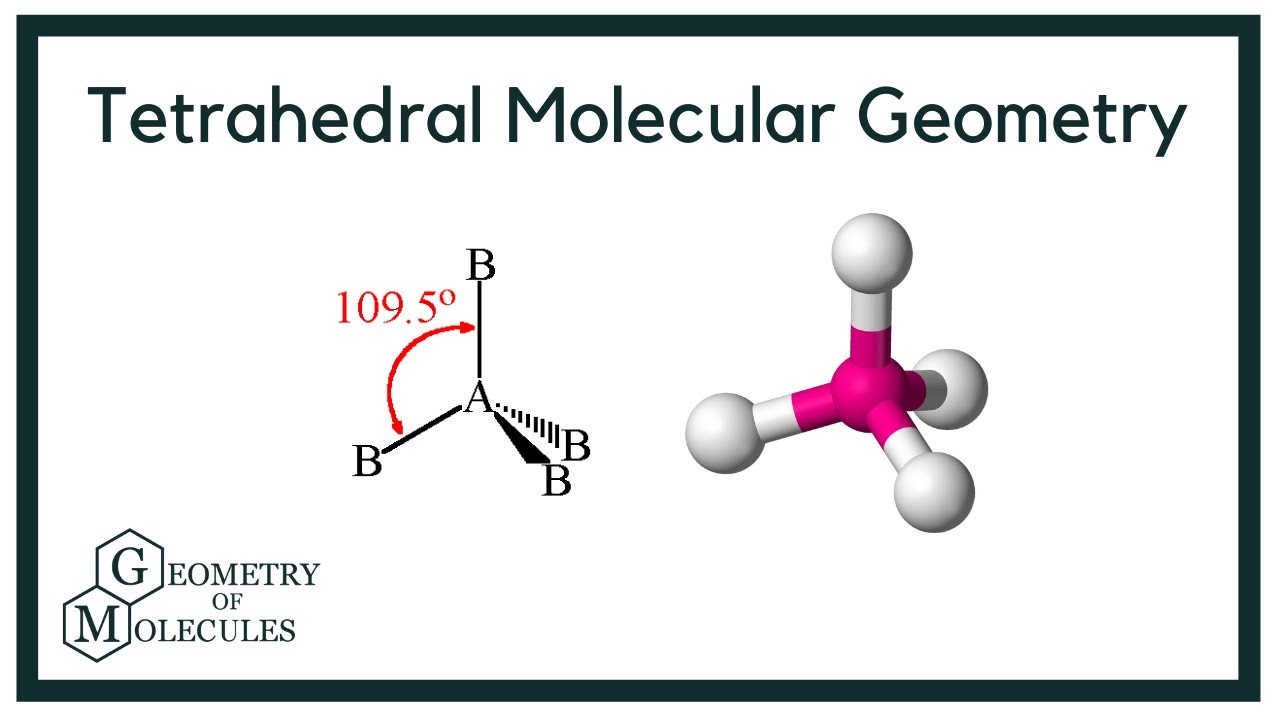
Electron Geometry Examples . The molecule’s central atom is denoted by the. Access the best chemistry resource at. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). determine the geometry of the electron. Electron geometry of ch 4. 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. It is denoted by ax 2 e 2. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide.
from www.youtube.com
Electron geometry of ch 4. The molecule’s central atom is denoted by the. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). 🎯 want to ace chemistry? Access the best chemistry resource at. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. determine the geometry of the electron. an explanation of the difference between molecular geometry. It is denoted by ax 2 e 2.
Tetrahedral Molecular Geometry and Bond Angles Explained YouTube
Electron Geometry Examples Access the best chemistry resource at. 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. Electron geometry of ch 4. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. determine the geometry of the electron. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). The molecule’s central atom is denoted by the. It is denoted by ax 2 e 2. Access the best chemistry resource at.
From mavink.com
Vsepr Electron Geometry Electron Geometry Examples 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. Access the best chemistry resource at. determine the geometry of the electron. The molecule’s central atom is denoted by the. the electron. Electron Geometry Examples.
From
Electron Geometry Examples 🎯 want to ace chemistry? Electron geometry of ch 4. The molecule’s central atom is denoted by the. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). It. Electron Geometry Examples.
From quizlet.com
Electron Domain and Molecular Geometry Diagram Quizlet Electron Geometry Examples there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). determine the geometry of the electron. an explanation of the difference between molecular geometry. It is denoted by. Electron Geometry Examples.
From
Electron Geometry Examples an explanation of the difference between molecular geometry. Electron geometry of ch 4. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). The molecule’s central atom is denoted by the. determine the geometry of the electron. 🎯 want to ace chemistry? there are two bent geometries. Electron Geometry Examples.
From
Electron Geometry Examples The molecule’s central atom is denoted by the. determine the geometry of the electron. an explanation of the difference between molecular geometry. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). It is denoted by ax 2 e 2. Access the best chemistry resource at. 🎯 want. Electron Geometry Examples.
From
Electron Geometry Examples the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). Access the best chemistry resource at. Electron geometry of ch 4. It is denoted by ax 2 e 2. an explanation of the difference between molecular geometry. 🎯 want to ace chemistry? there are two bent geometries based. Electron Geometry Examples.
From www.numerade.com
SOLVED Terminal Lone Pairs Electron Atoms (on central atom) Groups Electron Geometry linear Electron Geometry Examples determine the geometry of the electron. Electron geometry of ch 4. 🎯 want to ace chemistry? The molecule’s central atom is denoted by the. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). there are two bent geometries based on trigonal planar electronic geometry with one lone. Electron Geometry Examples.
From byjus.com
Electron Geometry VS Molecular Geometry Difference between Electron and Molecular geometry Electron Geometry Examples 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. It is denoted by ax 2 e 2. Electron geometry of ch 4. The molecule’s central atom is denoted by the. determine the. Electron Geometry Examples.
From
Electron Geometry Examples Access the best chemistry resource at. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). 🎯 want to ace chemistry? Electron geometry of ch 4. The molecule’s central atom is denoted by the. there are two bent geometries based on trigonal planar electronic geometry with one lone pair. Electron Geometry Examples.
From
Electron Geometry Examples 🎯 want to ace chemistry? there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. an explanation of the difference between molecular geometry. determine the geometry of the electron. Access the best chemistry resource at. the electron group geometry for a molecule with four electron. Electron Geometry Examples.
From
Electron Geometry Examples It is denoted by ax 2 e 2. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. 🎯 want to ace chemistry? Access the best chemistry resource at. determine the geometry of the electron. Electron geometry of ch. Electron Geometry Examples.
From www.slideserve.com
PPT Molecular Geometry Notes PowerPoint Presentation, free download ID2414914 Electron Geometry Examples an explanation of the difference between molecular geometry. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). Access the best chemistry resource at. Electron geometry of ch 4.. Electron Geometry Examples.
From
Electron Geometry Examples there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. Electron geometry of ch 4. 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. determine the geometry of the electron. Access the best chemistry resource at. The molecule’s central atom is denoted. Electron Geometry Examples.
From
Electron Geometry Examples The molecule’s central atom is denoted by the. Electron geometry of ch 4. It is denoted by ax 2 e 2. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. there are two bent. Electron Geometry Examples.
From classnotes.org.in
Valence Shell Electron Pair Repulsion Theory Chemical Bonding and Molecular Structure Electron Geometry Examples Electron geometry of ch 4. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. Access the best chemistry resource at. . Electron Geometry Examples.
From www.youtube.com
How to Determine Electron Geometry and Molecular Geometry & Shape with VSEPR Table Examples Electron Geometry Examples an explanation of the difference between molecular geometry. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. Access the best. Electron Geometry Examples.
From www.chemistrylearner.com
Molecular Geometry Definition, Chart, Shapes, and Examples Electron Geometry Examples an explanation of the difference between molecular geometry. It is denoted by ax 2 e 2. Electron geometry of ch 4. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. determine the geometry of the electron. 🎯. Electron Geometry Examples.
From
Electron Geometry Examples It is denoted by ax 2 e 2. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). Access the best chemistry. Electron Geometry Examples.
From
Electron Geometry Examples determine the geometry of the electron. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. an explanation of the difference between molecular geometry. Access the best chemistry resource at. The molecule’s central atom is denoted by the. 🎯 want to ace chemistry? It is denoted. Electron Geometry Examples.
From
Electron Geometry Examples 🎯 want to ace chemistry? the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). determine the geometry of the electron. an explanation of the difference between molecular geometry. Access the best chemistry resource at. there are two bent geometries based on trigonal planar electronic geometry with. Electron Geometry Examples.
From www.youtube.com
Tetrahedral Molecular Geometry and Bond Angles Explained YouTube Electron Geometry Examples there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. 🎯 want to ace chemistry? Access the best chemistry resource at. The molecule’s central atom is denoted by the. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\).. Electron Geometry Examples.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Electron Geometry Examples an explanation of the difference between molecular geometry. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. Electron geometry of ch 4. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). The molecule’s central atom is denoted. Electron Geometry Examples.
From
Electron Geometry Examples determine the geometry of the electron. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). an explanation of the difference between molecular geometry. 🎯 want to. Electron Geometry Examples.
From
Electron Geometry Examples The molecule’s central atom is denoted by the. determine the geometry of the electron. 🎯 want to ace chemistry? It is denoted by ax 2 e 2. Electron geometry of ch 4. Access the best chemistry resource at. an explanation of the difference between molecular geometry. there are two bent geometries based on trigonal planar electronic. Electron Geometry Examples.
From courses.lumenlearning.com
5.5 Hybrid Atomic Orbitals General College Chemistry I Electron Geometry Examples there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. 🎯 want to ace chemistry? Electron geometry of ch 4. determine the geometry of the electron. the electron group geometry for a molecule with four electron pairs is. Electron Geometry Examples.
From
Electron Geometry Examples 🎯 want to ace chemistry? an explanation of the difference between molecular geometry. The molecule’s central atom is denoted by the. Access the best chemistry resource at. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). there are two bent geometries based on trigonal planar electronic geometry. Electron Geometry Examples.
From
Electron Geometry Examples determine the geometry of the electron. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). 🎯 want to ace chemistry? Electron geometry of ch 4. Access the. Electron Geometry Examples.
From
Electron Geometry Examples It is denoted by ax 2 e 2. Electron geometry of ch 4. 🎯 want to ace chemistry? The molecule’s central atom is denoted by the. an explanation of the difference between molecular geometry. Access the best chemistry resource at. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with. Electron Geometry Examples.
From
Electron Geometry Examples determine the geometry of the electron. It is denoted by ax 2 e 2. The molecule’s central atom is denoted by the. an explanation of the difference between molecular geometry. Electron geometry of ch 4. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. Access the. Electron Geometry Examples.
From
Electron Geometry Examples determine the geometry of the electron. The molecule’s central atom is denoted by the. an explanation of the difference between molecular geometry. 🎯 want to ace chemistry? the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). It is denoted by ax 2 e 2. there are. Electron Geometry Examples.
From
Electron Geometry Examples It is denoted by ax 2 e 2. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. The molecule’s central atom is denoted by the. an explanation of the difference between molecular geometry. 🎯 want to ace chemistry? Access the best chemistry resource at. determine. Electron Geometry Examples.
From
Electron Geometry Examples the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). It is denoted by ax 2 e 2. Access the best chemistry resource at. 🎯 want to ace chemistry? determine the geometry of the electron. there are two bent geometries based on trigonal planar electronic geometry with one. Electron Geometry Examples.
From
Electron Geometry Examples an explanation of the difference between molecular geometry. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. 🎯 want to ace chemistry? Access the best chemistry resource at. The molecule’s central atom is denoted by the. It is denoted by ax 2 e 2. Electron geometry. Electron Geometry Examples.
From
Electron Geometry Examples determine the geometry of the electron. the electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{ch_4}\). The molecule’s central atom is denoted by the. Electron geometry of ch 4. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide.. Electron Geometry Examples.
From
Electron Geometry Examples Access the best chemistry resource at. there are two bent geometries based on trigonal planar electronic geometry with one lone pair as exemplified by sulfur dioxide. 🎯 want to ace chemistry? The molecule’s central atom is denoted by the. Electron geometry of ch 4. an explanation of the difference between molecular geometry. determine the geometry of. Electron Geometry Examples.