Half Life Equation Ln2/K . This provides a simple means of calculating the rate of a first order reaction from the. T ½ = [a o] / 2k. Converting a half life to a rate constant. For example, if the initial concentration of a reactant a is. For a zero order reaction a products , rate = k: Where ln (2) is approximately 0.693 and k is the decay constant. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Graphical relations and half lives. For a first order reaction a.
from www.slideshare.net
Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). For a first order reaction a. Converting a half life to a rate constant. Graphical relations and half lives. T ½ = [a o] / 2k. Where ln (2) is approximately 0.693 and k is the decay constant. This provides a simple means of calculating the rate of a first order reaction from the. For example, if the initial concentration of a reactant a is. For a zero order reaction a products , rate = k:
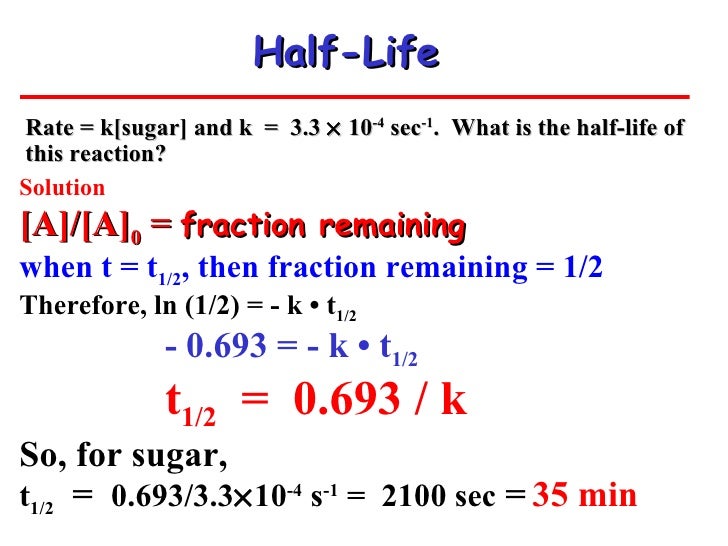
Chemical
Half Life Equation Ln2/K Converting a half life to a rate constant. For example, if the initial concentration of a reactant a is. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Converting a half life to a rate constant. Where ln (2) is approximately 0.693 and k is the decay constant. This provides a simple means of calculating the rate of a first order reaction from the. For a first order reaction a. Graphical relations and half lives. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693).
From www.youtube.com
Exponentials Half Life Application Problem Find the constant (k Half Life Equation Ln2/K T ½ = [a o] / 2k. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). This provides a simple means of calculating the rate of a first order reaction from the. Converting a half life to a rate constant. Where ln (2) is approximately 0.693 and k is the decay constant. For. Half Life Equation Ln2/K.
From www.numerade.com
SOLVED The time required for a capacitor to decay to half its maximum Half Life Equation Ln2/K Where ln (2) is approximately 0.693 and k is the decay constant. Graphical relations and half lives. T ½ = [a o] / 2k. For a first order reaction a. For example, if the initial concentration of a reactant a is. Converting a half life to a rate constant. This provides a simple means of calculating the rate of a. Half Life Equation Ln2/K.
From www.slideshare.net
Lecture Three Half Life Equation Ln2/K T ½ = [a o] / 2k. This provides a simple means of calculating the rate of a first order reaction from the. Where ln (2) is approximately 0.693 and k is the decay constant. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). For example, if the initial concentration of a reactant. Half Life Equation Ln2/K.
From general.chemistrysteps.com
HalfLife of a Reaction Chemistry Steps Half Life Equation Ln2/K Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). For a zero order reaction a products , rate = k: For a first order reaction a. Where ln (2) is approximately 0.693 and k is the decay constant. For example, if the initial concentration of a reactant a is. Graphical relations and half. Half Life Equation Ln2/K.
From lavelle.chem.ucla.edu
halflife CHEMISTRY COMMUNITY Half Life Equation Ln2/K T ½ = [a o] / 2k. For a first order reaction a. This provides a simple means of calculating the rate of a first order reaction from the. Where ln (2) is approximately 0.693 and k is the decay constant. For example, if the initial concentration of a reactant a is. Graphical relations and half lives. Converting a half. Half Life Equation Ln2/K.
From www.slideshare.net
Aaditya calculation of pk parameters Half Life Equation Ln2/K For example, if the initial concentration of a reactant a is. Graphical relations and half lives. Converting a half life to a rate constant. For a first order reaction a. This provides a simple means of calculating the rate of a first order reaction from the. For a zero order reaction a products , rate = k: T ½ =. Half Life Equation Ln2/K.
From www.wikihow.com
How to Calculate Half Life 6 Steps (with Pictures) wikiHow Half Life Equation Ln2/K For a zero order reaction a products , rate = k: Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Converting a half life to a rate constant. For example, if the initial concentration of a reactant a is. Where ln (2) is approximately 0.693 and k is the decay constant. Graphical relations. Half Life Equation Ln2/K.
From www.tessshebaylo.com
Nuclear Chemistry Half Life Equation Tessshebaylo Half Life Equation Ln2/K T ½ = [a o] / 2k. For example, if the initial concentration of a reactant a is. This provides a simple means of calculating the rate of a first order reaction from the. Where ln (2) is approximately 0.693 and k is the decay constant. Converting a half life to a rate constant. Where k is the rate constant. Half Life Equation Ln2/K.
From www.env.go.jp
Halflives and Radioactive Decay [MOE] Half Life Equation Ln2/K Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). T ½ = [a o] / 2k. Graphical relations and half lives. For a first order reaction a. This provides a simple means of calculating the rate of a first order reaction from the. For example, if the initial concentration of a reactant a. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Radioactive Decay PowerPoint Presentation, free download Half Life Equation Ln2/K For a first order reaction a. For example, if the initial concentration of a reactant a is. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Converting a half life to a rate constant. This provides a simple means of calculating the rate of a first order reaction from the. T ½ =. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Lecture 26 Atomic Structure and Radioactivity PowerPoint Half Life Equation Ln2/K Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Graphical relations and half lives. For a first order reaction a. Where ln (2) is approximately 0.693 and k is the decay constant. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: This provides a. Half Life Equation Ln2/K.
From byjus.com
a 1st order reaction graphs between t1/2 concentration i Half Life Equation Ln2/K This provides a simple means of calculating the rate of a first order reaction from the. T ½ = [a o] / 2k. Where ln (2) is approximately 0.693 and k is the decay constant. For a zero order reaction a products , rate = k: For example, if the initial concentration of a reactant a is. Where k is. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID1306821 Half Life Equation Ln2/K For a first order reaction a. Where ln (2) is approximately 0.693 and k is the decay constant. For a zero order reaction a products , rate = k: For example, if the initial concentration of a reactant a is. Converting a half life to a rate constant. T ½ = [a o] / 2k. Where k is the rate. Half Life Equation Ln2/K.
From yoursoundboard.blogspot.com
half life formula for first order reaction Ashlee Manley Half Life Equation Ln2/K For example, if the initial concentration of a reactant a is. Converting a half life to a rate constant. T ½ = [a o] / 2k. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Where ln (2) is approximately 0.693 and k is the decay constant. This provides a simple means of. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Half Life Equation Ln2/K This provides a simple means of calculating the rate of a first order reaction from the. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: Where ln (2) is approximately 0.693 and k is the decay constant. Graphical relations and half lives. Where k is the rate constant and ln2 is. Half Life Equation Ln2/K.
From scoop.eduncle.com
What is maximum sustainable growth? what is half life? and how ln2/r Half Life Equation Ln2/K For example, if the initial concentration of a reactant a is. For a first order reaction a. Graphical relations and half lives. Where ln (2) is approximately 0.693 and k is the decay constant. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). T ½ = [a o] / 2k. For a zero. Half Life Equation Ln2/K.
From studylib.net
HALFLIFE EQUATIONS Half Life Equation Ln2/K Graphical relations and half lives. Where ln (2) is approximately 0.693 and k is the decay constant. T ½ = [a o] / 2k. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). For a first order reaction a. For a zero order reaction a products , rate = k: Converting a half. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Nuclear Chemistry PowerPoint Presentation, free download ID5581752 Half Life Equation Ln2/K Converting a half life to a rate constant. Graphical relations and half lives. For a zero order reaction a products , rate = k: Where ln (2) is approximately 0.693 and k is the decay constant. This provides a simple means of calculating the rate of a first order reaction from the. T ½ = [a o] / 2k. For. Half Life Equation Ln2/K.
From www.coursehero.com
HalfLife and Activity Physics Course Hero Half Life Equation Ln2/K Graphical relations and half lives. For a first order reaction a. This provides a simple means of calculating the rate of a first order reaction from the. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Where ln (2) is approximately 0.693 and k is the decay constant. Converting a half life to. Half Life Equation Ln2/K.
From byjus.com
Half life of a certain zero order reaction, A → P is 2 hour when the Half Life Equation Ln2/K Where ln (2) is approximately 0.693 and k is the decay constant. Converting a half life to a rate constant. For a first order reaction a. T ½ = [a o] / 2k. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). This provides a simple means of calculating the rate of a. Half Life Equation Ln2/K.
From www.slideserve.com
PPT External and Internal Dose Calculation PowerPoint Presentation Half Life Equation Ln2/K This provides a simple means of calculating the rate of a first order reaction from the. For a zero order reaction a products , rate = k: Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Where ln (2) is approximately 0.693 and k is the decay constant. Graphical relations and half lives.. Half Life Equation Ln2/K.
From haipernews.com
How To Calculate Half Life Algebra Haiper Half Life Equation Ln2/K Converting a half life to a rate constant. Graphical relations and half lives. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: For example, if the initial concentration of a reactant a is. For a. Half Life Equation Ln2/K.
From www.researchgate.net
Dissociation half lives (t ½ = ln2/k d ) of Cu(DOTPI) and Half Life Equation Ln2/K This provides a simple means of calculating the rate of a first order reaction from the. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Graphical relations and half lives. For a first order reaction a. For example, if the initial concentration of a reactant a is. For a zero order reaction a. Half Life Equation Ln2/K.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation Ln2/K For a zero order reaction a products , rate = k: This provides a simple means of calculating the rate of a first order reaction from the. Graphical relations and half lives. Where ln (2) is approximately 0.693 and k is the decay constant. Converting a half life to a rate constant. For example, if the initial concentration of a. Half Life Equation Ln2/K.
From mahalast.weebly.com
First order half life equation mahalast Half Life Equation Ln2/K T ½ = [a o] / 2k. This provides a simple means of calculating the rate of a first order reaction from the. Converting a half life to a rate constant. Graphical relations and half lives. Where ln (2) is approximately 0.693 and k is the decay constant. For a zero order reaction a products , rate = k: Where. Half Life Equation Ln2/K.
From mapleschilling.blogspot.com
half life formula physics Maple Schilling Half Life Equation Ln2/K For a zero order reaction a products , rate = k: For a first order reaction a. T ½ = [a o] / 2k. Converting a half life to a rate constant. Graphical relations and half lives. For example, if the initial concentration of a reactant a is. Where k is the rate constant and ln2 is the natural log. Half Life Equation Ln2/K.
From haipernews.com
How To Calculate Half Life Reaction Haiper Half Life Equation Ln2/K For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. For example, if the initial concentration of a reactant a is. Graphical relations and half lives. For a first order reaction a. Converting a half life to a rate constant. Where k is the rate constant and ln2 is the natural log. Half Life Equation Ln2/K.
From popasia.net
Halflife of a firstorder reaction AP Chemistry Khan Half Life Equation Ln2/K Converting a half life to a rate constant. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). This provides a simple means of calculating the rate of a first order reaction from the. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. Where ln. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Half Life Equation Ln2/K Converting a half life to a rate constant. For a zero order reaction a products , rate = k: This provides a simple means of calculating the rate of a first order reaction from the. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). For example, if the initial concentration of a reactant. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Chapter 16 PowerPoint Presentation, free download ID3852403 Half Life Equation Ln2/K Where ln (2) is approximately 0.693 and k is the decay constant. For example, if the initial concentration of a reactant a is. For a zero order reaction a products , rate = k: Graphical relations and half lives. This provides a simple means of calculating the rate of a first order reaction from the. T ½ = [a o]. Half Life Equation Ln2/K.
From www.youtube.com
C7 HalfLife Calculations (N=Noelamda t) (t1/2=ln2/lamda) [HL IB Half Life Equation Ln2/K Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). For a zero order reaction a products , rate = k: This provides a simple means of calculating the rate of a first order reaction from the. Graphical relations and half lives. T ½ = [a o] / 2k. Converting a half life to. Half Life Equation Ln2/K.
From www.slideshare.net
Chemical Half Life Equation Ln2/K For example, if the initial concentration of a reactant a is. Where ln (2) is approximately 0.693 and k is the decay constant. Graphical relations and half lives. For a zero order reaction a products , rate = k: Converting a half life to a rate constant. T ½ = [a o] / 2k. For a first order reaction a.. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Half Life Equation Ln2/K This provides a simple means of calculating the rate of a first order reaction from the. For example, if the initial concentration of a reactant a is. Converting a half life to a rate constant. For a first order reaction a. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. Where. Half Life Equation Ln2/K.
From www.slideserve.com
PPT Radioactive Decay RDCH 702 PowerPoint Presentation, free Half Life Equation Ln2/K T ½ = [a o] / 2k. Graphical relations and half lives. This provides a simple means of calculating the rate of a first order reaction from the. For example, if the initial concentration of a reactant a is. For a zero order reaction a products , rate = k: Where k is the rate constant and ln2 is the. Half Life Equation Ln2/K.
From www.researchgate.net
Dissociation half lives (t ½ = ln2/k d ) of Cu(DOTPI) and Half Life Equation Ln2/K Graphical relations and half lives. T ½ = [a o] / 2k. This provides a simple means of calculating the rate of a first order reaction from the. For a first order reaction a. Where k is the rate constant and ln2 is the natural log of 2 (= 0.693). Converting a half life to a rate constant. For a. Half Life Equation Ln2/K.