Table Salt Observation . Put several grains of salt on a microscope slide. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Stir the mixture gently for. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Strong electrolytes include soluble ionic compounds and strong acids. Examples of qualitative observations include the following: Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. The outside air temperature is cooler during the winter season,. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they.
from keplarllp.com
Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Put several grains of salt on a microscope slide. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. The outside air temperature is cooler during the winter season,. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Examples of qualitative observations include the following: Stir the mixture gently for. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Strong electrolytes include soluble ionic compounds and strong acids.
️ How to calculate percentage purity of an impure sample. How to
Table Salt Observation Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. The outside air temperature is cooler during the winter season,. Strong electrolytes include soluble ionic compounds and strong acids. Put several grains of salt on a microscope slide. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Examples of qualitative observations include the following: Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Stir the mixture gently for.
From byjus.com
How to memorize the colour of all the salts???? Table Salt Observation Put several grains of salt on a microscope slide. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Strong electrolytes dissociate. Table Salt Observation.
From handletheheat.com
Kosher Salt vs. Sea Salt vs. Table Salt Handle the Heat Table Salt Observation Put several grains of salt on a microscope slide. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Small amounts of sodium aluminosilicate, tricalcium. Table Salt Observation.
From www.alamy.com
Regular Table Salt. Isolated on white background Stock Photo Alamy Table Salt Observation The outside air temperature is cooler during the winter season,. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Examples of qualitative observations include the following: Stir the mixture gently for. Strong electrolytes include soluble ionic compounds and strong acids. Students will dissolve various salts into water, make predictions about what will happen when the solutions are. Table Salt Observation.
From www.researchgate.net
A log of all the SALT observations. The Hα equivalent width Table Salt Observation Strong electrolytes include soluble ionic compounds and strong acids. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to. Table Salt Observation.
From www.chegg.com
Solved 2. Table salt has a molecular formula of NaCl (i.e. 1 Table Salt Observation The outside air temperature is cooler during the winter season,. Strong electrolytes include soluble ionic compounds and strong acids. Examples of qualitative observations include the following: Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make. Table Salt Observation.
From www.slideserve.com
PPT Chapter 3 Matter and Energy PowerPoint Presentation, free Table Salt Observation Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Put several grains of salt on a microscope slide. The outside air temperature is cooler during the winter season,. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. If you use. Table Salt Observation.
From www.space.com
Meteorite contaminated with table salt after landing on Earth Space Table Salt Observation Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Students will dissolve various salts into water, make predictions about what will happen when the solutions. Table Salt Observation.
From www.studypool.com
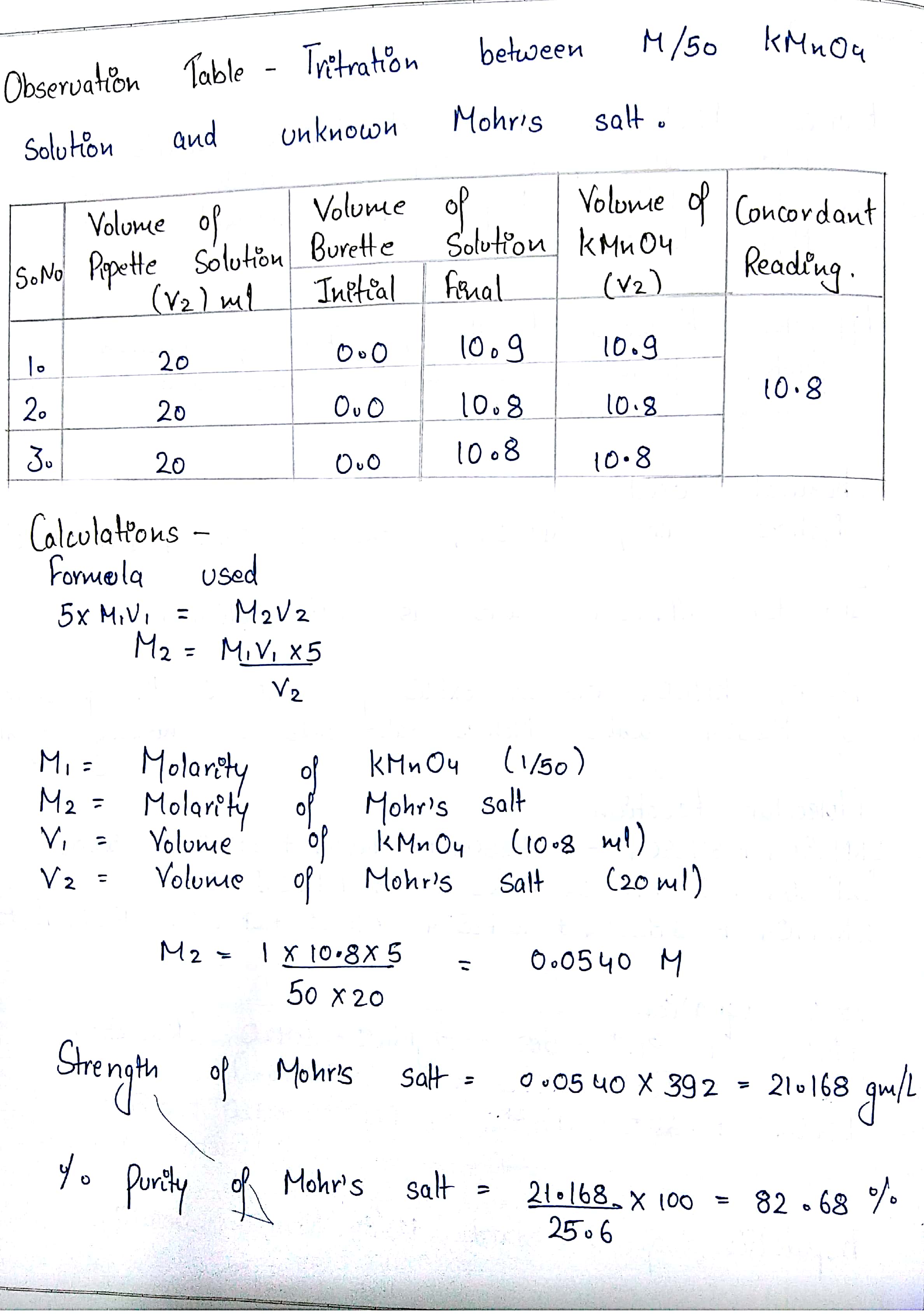
SOLUTION Strength of mohr s salt vs kmno4 volumetric analysis Studypool Table Salt Observation Strong electrolytes include soluble ionic compounds and strong acids. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Stir the mixture gently for. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left. Table Salt Observation.
From studylib.net
Properties of table salt Table Salt Observation Stir the mixture gently for. Examples of qualitative observations include the following: Strong electrolytes include soluble ionic compounds and strong acids. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. The outside air temperature is cooler during the winter season,. Students will dissolve various salts into water, make. Table Salt Observation.
From www.studocu.com
Salt No.1 (Ammonium Carbonate) QUALITATIVE ANALYSIS SALT NO Table Salt Observation Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Strong electrolytes include soluble ionic compounds and strong acids. The outside air temperature is cooler during. Table Salt Observation.
From garlicdelight.com
How to season to taste 13 tips for salting correctly Table Salt Observation Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Stir the mixture gently for. Examples of. Table Salt Observation.
From www.researchgate.net
(PDF) Why Is There Iodine in Table Salt? Table Salt Observation Examples of qualitative observations include the following: Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. If you use. Table Salt Observation.
From www.studocu.com
Salt no. 7 ammonium sulphate SALT NO. 7 EXPERIMENT OBSERVATION Table Salt Observation Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Examples of qualitative observations include the following: Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Put several grains of salt on a microscope slide. Stir the mixture. Table Salt Observation.
From www.coursehero.com
[Solved] Table salt contains 39.33 g of sodium per 100 g of salt. The U Table Salt Observation Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Strong electrolytes include soluble ionic compounds and strong acids. Put several grains of salt on a microscope slide. Examples of qualitative observations include the following: Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom. Table Salt Observation.
From demarosalt.com
Himalayan Salt vs. Table Salt Uncover Key Differences & Benefits Table Salt Observation Stir the mixture gently for. Put several grains of salt on a microscope slide. Strong electrolytes include soluble ionic compounds and strong acids. The outside air temperature is cooler during the winter season,. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here,. Table Salt Observation.
From www.slideshare.net
CHEMISTRY Salt analysis class 12 Table Salt Observation Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Stir the mixture gently for. Put several grains of salt on a microscope slide. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. If you use a compound. Table Salt Observation.
From windsorsalt.com
WHAT IS TABLE SALT? Windsor Salt Table Salt Observation Stir the mixture gently for. The outside air temperature is cooler during the winter season,. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. If you use a compound microscope, put a coverslip on it (prevent it from scratching the.. Table Salt Observation.
From onlinecalculator.guru
Salt Analysis Formulas Complete List Formulae Sheet for Salt Analysis Table Salt Observation Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Put several grains of salt on a microscope slide. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Examples of qualitative observations include the following:. Table Salt Observation.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Observation Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. The outside air temperature is cooler during the winter season,. If you use a compound microscope,. Table Salt Observation.
From www.researchgate.net
Summary of SALT observations. Download Table Table Salt Observation The outside air temperature is cooler during the winter season,. Examples of qualitative observations include the following: Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond. Table Salt Observation.
From learnchemistrysaltform4.weebly.com
Qualitative analysis of salts Learn Chemistry Corner Table Salt Observation Examples of qualitative observations include the following: Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Stir the mixture gently for. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. If you use a compound microscope, put. Table Salt Observation.
From www.researchgate.net
2. Qualitative Observations of Salt Coverage for CSM Runs Download Table Table Salt Observation Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Strong electrolytes include soluble ionic compounds and strong acids. The outside air temperature is cooler during the winter season,. Put several grains of salt on a microscope slide. Stir the mixture gently for. Examples of qualitative observations include the following: Students will dissolve various salts into water, make. Table Salt Observation.
From www.dpreview.com
Video How photographic salt prints are made Digital Photography Review Table Salt Observation Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Strong electrolytes include soluble ionic compounds and strong acids. Examples of qualitative observations include the following: Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it forms results from the donation of an electron from one atom (here, na) to another (cl), rather. Put several grains. Table Salt Observation.
From adamcvean.wordpress.com
Under The Microscope Sea Salt VS Table Salt Ada McVean Table Salt Observation Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Stir the mixture gently for. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Examples of qualitative observations include the following: The outside air temperature is cooler during the winter season,. Put. Table Salt Observation.
From www.pdfprof.com
calculate the molarity of 1.60 l of a solution Table Salt Observation Examples of qualitative observations include the following: Put several grains of salt on a microscope slide. The outside air temperature is cooler during the winter season,. Strong electrolytes include soluble ionic compounds and strong acids. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. If you use a compound microscope, put a coverslip on it (prevent it. Table Salt Observation.
From www.youtube.com
Table Salt from the Elements YouTube Table Salt Observation Put several grains of salt on a microscope slide. Examples of qualitative observations include the following: Strong electrolytes dissociate completely into ions and thus carry relatively large currents. The outside air temperature is cooler during the winter season,. Strong electrolytes include soluble ionic compounds and strong acids. Stir the mixture gently for. Students will dissolve various salts into water, make. Table Salt Observation.
From www.alamy.com
Irregular crystals of sea salt, aka common salt or table salt (sodium Table Salt Observation Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Put several grains of salt on a microscope slide. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Strong. Table Salt Observation.
From gbu-presnenskij.ru
Sea Salt Table Salt Differences And Health Benefits, 58 OFF Table Salt Observation The outside air temperature is cooler during the winter season,. Stir the mixture gently for. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Examples of qualitative observations include the following: Strong electrolytes include soluble ionic compounds and strong acids. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Students will dissolve various. Table Salt Observation.
From www.todayifoundout.com
The Difference Between Kosher Salt and Table Salt Table Salt Observation Strong electrolytes include soluble ionic compounds and strong acids. Small amounts of sodium aluminosilicate, tricalcium phosphate, or magnesium. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Put. Table Salt Observation.
From keplarllp.com
️ How to calculate percentage purity of an impure sample. How to Table Salt Observation Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Strong electrolytes include soluble ionic compounds and strong acids. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Table salt (sodium chloride, or nacl). Table Salt Observation.
From www.studocu.com
SALT Analysis (2) oiuy SALT ANALYSIS EXPERIMENT OBSERVATION Table Salt Observation Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Stir the mixture gently for. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Strong electrolytes include soluble ionic compounds and strong acids. Small amounts of sodium aluminosilicate,. Table Salt Observation.
From yesdirt.com
Is Salt (ANSWERED) Yes Dirt Table Salt Observation If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. Table salt (sodium chloride, or nacl) is an ionic compound, meaning that the bond it. Table Salt Observation.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt Observation Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Strong electrolytes include soluble ionic compounds and strong acids. If you use a compound microscope, put a coverslip on it (prevent it from scratching the. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations. Table Salt Observation.
From www.attainable-sustainable.net
Canning Salt vs. Table Salt for Food Preservation Attainable Sustainable® Table Salt Observation Strong electrolytes include soluble ionic compounds and strong acids. Examples of qualitative observations include the following: The outside air temperature is cooler during the winter season,. Put several grains of salt on a microscope slide. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide. Table Salt Observation.
From www.canadiannaturephotographer.com
Crystals Photographed with Polarization Microscopy The Canadian Table Salt Observation Stir the mixture gently for. Students will dissolve various salts into water, make predictions about what will happen when the solutions are left to dry, then make observations and provide explanations about what they. The outside air temperature is cooler during the winter season,. Strong electrolytes dissociate completely into ions and thus carry relatively large currents. Examples of qualitative observations. Table Salt Observation.