Flame Test Nacl . In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. flame tests using metal salts. Then, place the wire in the flame just above the. It is widely used to detect. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. the flame test is one of the most commonly used analytical processes in chemistry. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment.
from www.chegg.com
the flame test is one of the most commonly used analytical processes in chemistry. Then, place the wire in the flame just above the. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. It is widely used to detect. flame tests using metal salts. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution.
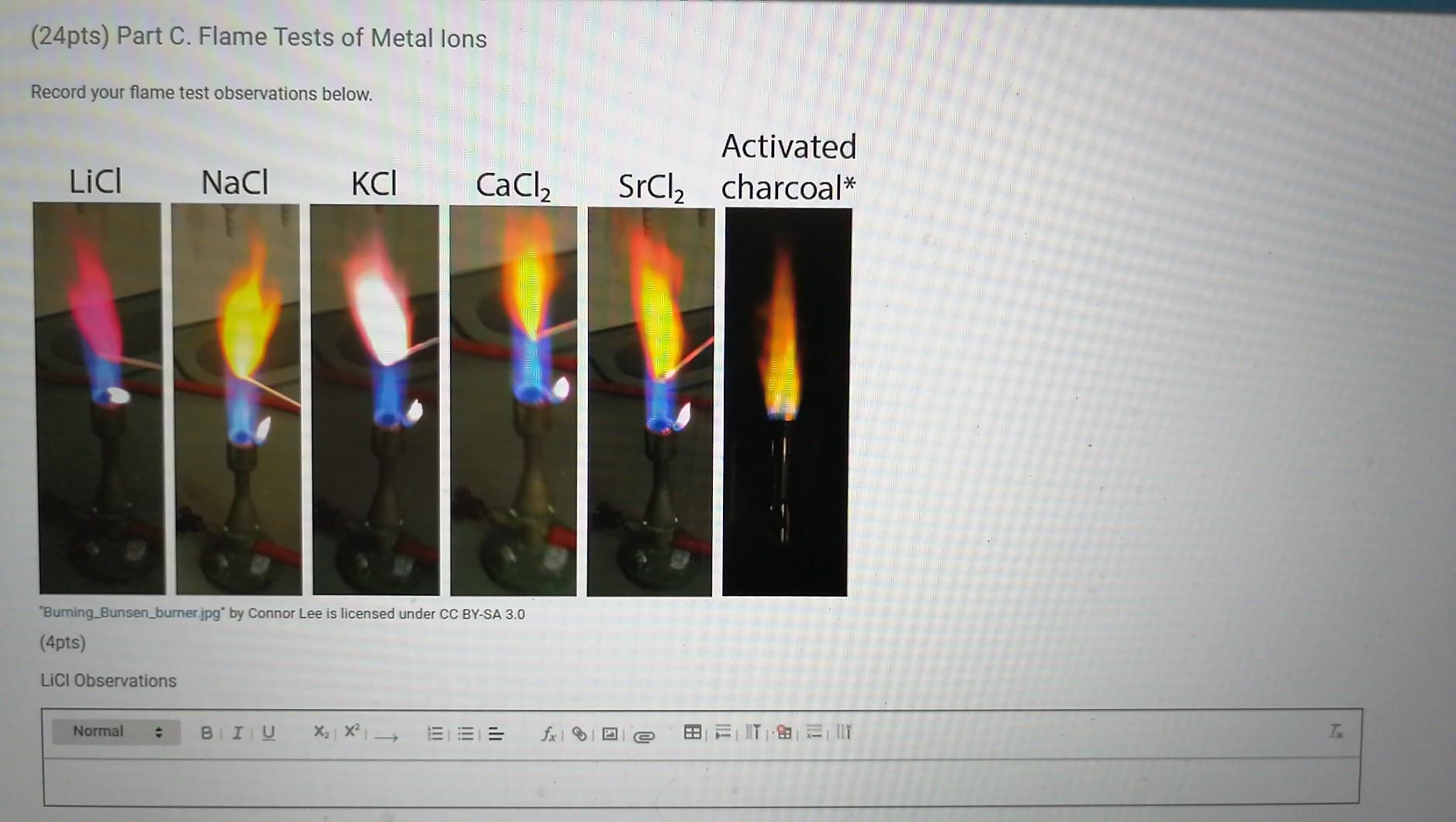
Solved (24pts) Part C. Flame Tests of Metal lons Record your
Flame Test Nacl the flame test is one of the most commonly used analytical processes in chemistry. the flame test is one of the most commonly used analytical processes in chemistry. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. It is widely used to detect. flame tests using metal salts. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. Then, place the wire in the flame just above the. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts.
From www.amazingrust.com
Amazing Flame Test Flame Test Nacl flame tests using metal salts. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. Then, place the wire in the flame just above the. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. the flame test is one of the most commonly used analytical processes in. Flame Test Nacl.
From www.numerade.com
SOLVED Flame Tests for (CaCl2, BaCl2, NaCl, LiCl, KCl, CuCl2, SrCl2 Flame Test Nacl In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. It is widely used to detect. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is one of the most. Flame Test Nacl.
From www.alamy.com
Sodium metal flame test a clean nichrome wire is dipped into a salt Flame Test Nacl In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. It is widely used to detect. Then, place the wire in the flame just above the. the flame test is one of the most commonly used analytical processes in chemistry. flame tests using metal salts. Help students. Flame Test Nacl.
From www.youtube.com
Potassium chloride flame test YouTube Flame Test Nacl the flame test is one of the most commonly used analytical processes in chemistry. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. flame tests using. Flame Test Nacl.
From fineartamerica.com
Flame Test For Strontium Chloride Photograph by Clive Streeter Flame Test Nacl now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. It is widely used to detect. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.. Flame Test Nacl.
From fineartamerica.com
Sodium Metal Flame Test Photograph by Andrew Mcclenaghan/science Photo Flame Test Nacl Then, place the wire in the flame just above the. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. It is widely used to detect. flame tests. Flame Test Nacl.
From www.sciencephoto.com
Sodium Chloride Flame Test Stock Image C022/0782 Science Photo Flame Test Nacl flame tests using metal salts. It is widely used to detect. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is one of the most commonly used analytical processes in chemistry. Help students to reveal the burning colour of splints. Flame Test Nacl.
From www.sciencephoto.com
Performing a sodium flame test Stock Image A510/0109 Science Flame Test Nacl this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is one of the most commonly used analytical processes in chemistry. It is widely used to detect. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. Help students. Flame Test Nacl.
From www.youtube.com
Sodium( NaCl) flame test YouTube Flame Test Nacl Then, place the wire in the flame just above the. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. flame tests using metal salts. this page describes how to perform a flame test for a range of. Flame Test Nacl.
From www.youtube.com
Flame test Sodium chloride YouTube Flame Test Nacl flame tests using metal salts. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how. Flame Test Nacl.
From fineartamerica.com
Sodium Flame Test Photograph by Andrew Lambert Photography Flame Test Nacl Then, place the wire in the flame just above the. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. It is widely used to detect. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. this page describes how to. Flame Test Nacl.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Flame Test Nacl Then, place the wire in the flame just above the. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. It is widely used to detect. Help students to reveal the burning colour of splints soaked. Flame Test Nacl.
From sciencephotogallery.com
Performing A Sodium Flame Test by Jerry Mason/science Photo Library Flame Test Nacl It is widely used to detect. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. the flame test is one of the most commonly used analytical processes in chemistry. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. Then, place the wire in the flame just above. Flame Test Nacl.
From www.youtube.com
Flame Test (Sodium Chloride) YouTube Flame Test Nacl In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the. Flame Test Nacl.
From fineartamerica.com
Sodium Flame Test Photograph by David Taylor/science Photo Library Flame Test Nacl In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. the flame test is one of the most commonly used analytical processes in chemistry. flame tests using metal salts. Then,. Flame Test Nacl.
From narodnatribuna.info
Sodium Flame Test Photograph By Science Photo Library Flame Test Nacl flame tests using metal salts. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. the flame test is one of the most commonly used analytical processes in chemistry. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. this page describes how. Flame Test Nacl.
From fineartamerica.com
Sodium Flame Test Photograph by Science Photo Library Fine Art America Flame Test Nacl It is widely used to detect. flame tests using metal salts. the flame test is one of the most commonly used analytical processes in chemistry. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. this page describes how to perform a flame test for a. Flame Test Nacl.
From www.slideserve.com
PPT NaCl Find LE f PowerPoint Presentation, free download ID3253990 Flame Test Nacl Then, place the wire in the flame just above the. It is widely used to detect. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. flame tests using metal salts. this page describes how to perform a. Flame Test Nacl.
From www.scribd.com
Cfns Experiment 79 Flame Tests (Wooden Splint Method) 2 PDF Flame Test Nacl now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. flame tests using metal salts. Then, place the wire in the flame just above the. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. It is widely used to detect. Help students to reveal. Flame Test Nacl.
From www.coursehero.com
[Solved] Flame test (LiCl, KCl, NaCl, CaCl2) 30 points Introduction The Flame Test Nacl the flame test is one of the most commonly used analytical processes in chemistry. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts.. Flame Test Nacl.
From www.sciencephoto.com
Flame test on sodium Stock Image A510/0229 Science Photo Library Flame Test Nacl Then, place the wire in the flame just above the. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is one of the most commonly used analytical processes in chemistry. now, using nacl(aq) solution, dip the nichrome wire in the. Flame Test Nacl.
From www.youtube.com
Qualitative Flame Test NaCl Sodium Chloride YouTube Flame Test Nacl It is widely used to detect. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Then, place the wire in the flame just above the. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal. Flame Test Nacl.
From aidensdp.weebly.com
Flame Test Flame Test Nacl Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. It is widely used to detect. Then, place the wire in the flame just above the. the flame test is one of the most commonly used analytical processes in chemistry. this page describes how to perform a flame test for a. Flame Test Nacl.
From chem.libretexts.org
Flame Tests Chemistry LibreTexts Flame Test Nacl this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Then, place the wire in the flame just above the. the flame test is one of the most commonly used analytical processes in chemistry. Help students to reveal the burning colour of splints soaked in. Flame Test Nacl.
From www.researchgate.net
Flame test experiments involving control aqueous solutions consistingo Flame Test Nacl It is widely used to detect. Then, place the wire in the flame just above the. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. flame tests using metal salts. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. In. Flame Test Nacl.
From www.alamy.com
FLAME TEST SODIUM CHLORIDE SHOWING YELLOW ELEMENTAL FLUORESCENCE AND Flame Test Nacl flame tests using metal salts. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. It is widely used to detect. Then, place the wire in the flame just above the. . Flame Test Nacl.
From es.scribd.com
FLAME TESTS Lesson 17 Technicolor Atoms Sodium Sodium Chloride Flame Test Nacl now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. Then, place the wire in the flame just above the. flame tests using metal salts. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. the flame test is one of the most commonly used analytical processes in. Flame Test Nacl.
From ar.inspiredpencil.com
Flame Test Sodium Flame Test Nacl Then, place the wire in the flame just above the. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. flame tests using metal salts. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color. Flame Test Nacl.
From www.chegg.com
Solved (24pts) Part C. Flame Tests of Metal lons Record your Flame Test Nacl In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. It is widely used to detect. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Help students to reveal the burning colour of splints. Flame Test Nacl.
From www.thoughtco.com
How to Do a Flame Test for Qualitative Analysis Flame Test Nacl Then, place the wire in the flame just above the. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. this page describes how to perform a flame test for a. Flame Test Nacl.
From sciencephotogallery.com
Sodium Flame Test 1 by Science Photo Library Flame Test Nacl this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. It is widely used to detect. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. now, using nacl(aq) solution, dip the nichrome wire. Flame Test Nacl.
From issr.edu.kh
Flame tests Flame Test Nacl Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. In this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. It is widely used to detect. Then, place the. Flame Test Nacl.
From www.coursehero.com
[Solved] Flame test (LiCl, KCl, NaCl, CaCl2) 30 points Introduction The Flame Test Nacl the flame test is one of the most commonly used analytical processes in chemistry. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. It is widely used to detect. flame tests using metal salts. Then, place the wire in the flame just above. Flame Test Nacl.
From www.alamy.com
Flame experiment holding sodium chloride compound on platinum wire in Flame Test Nacl It is widely used to detect. the flame test is one of the most commonly used analytical processes in chemistry. flame tests using metal salts. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. now, using nacl(aq) solution, dip the nichrome wire. Flame Test Nacl.
From www.alamy.com
Flame Test Bunsen Burner Stock Photos & Flame Test Bunsen Burner Stock Flame Test Nacl Then, place the wire in the flame just above the. now, using nacl(aq) solution, dip the nichrome wire in the cationic solution. Help students to reveal the burning colour of splints soaked in different chloride solutions in this experiment. flame tests using metal salts. In this classic science experiment, students report on the colours produced when flame tests. Flame Test Nacl.