Experiment Using Catalysts . Catalysts provide an alternative route for reactions to proceed. The catalase enzyme (found in blood) lowers the. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. A catalyst gets reactions started and makes them happen faster by. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst.
from www.mdpi.com
Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. A catalyst gets reactions started and makes them happen faster by. The catalase enzyme (found in blood) lowers the. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. Catalysts provide an alternative route for reactions to proceed.
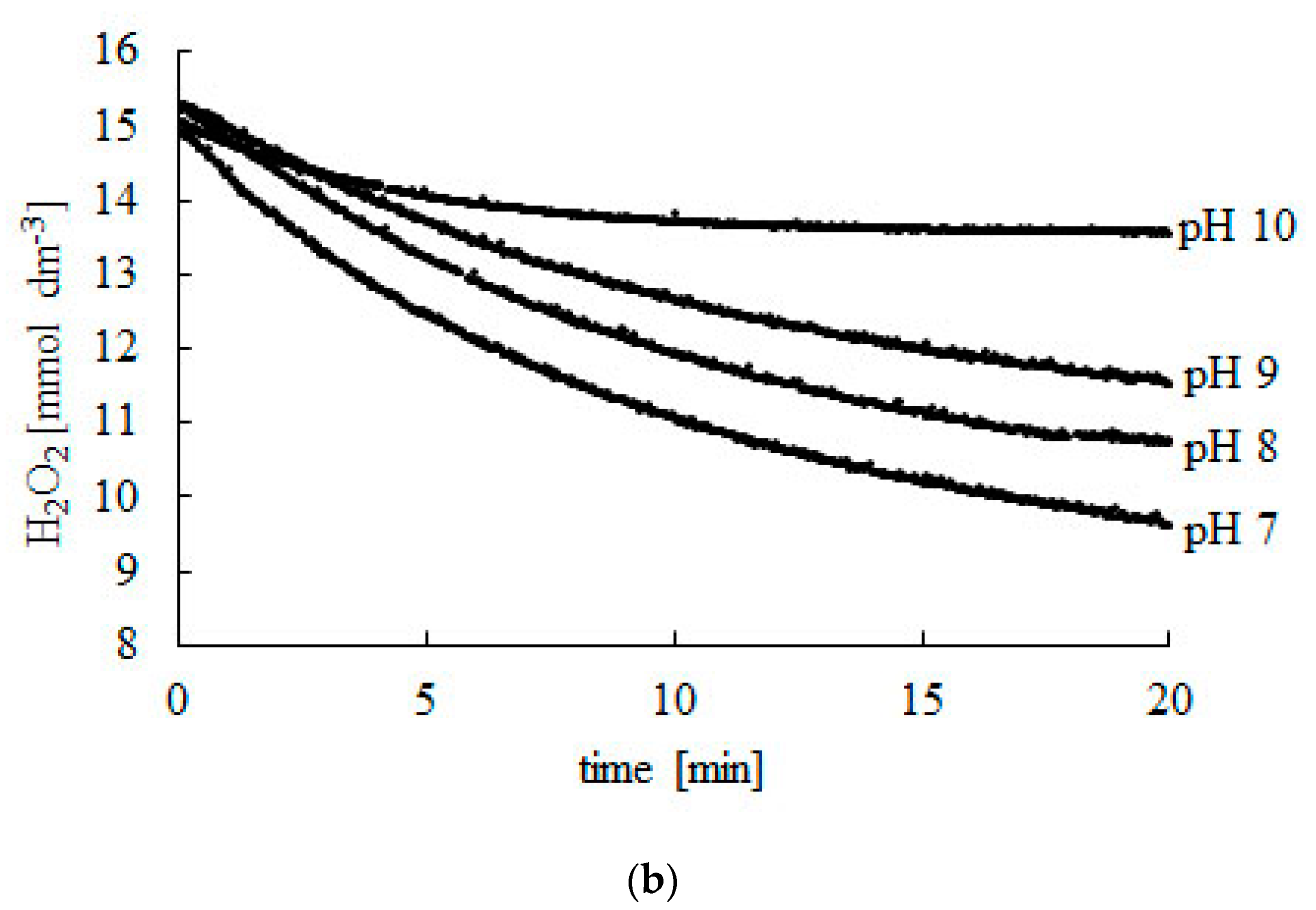
Catalysts Free FullText New Method of Determining Parameters for of
Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalysts provide an alternative route for reactions to proceed. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. A catalyst gets reactions started and makes them happen faster by. Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. The catalase enzyme (found in blood) lowers the. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates.
From www.youtube.com
Catalysts + H2O2 demonstration EXPERIMENT YouTube Experiment Using Catalysts In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. Catalysts provide an alternative route for reactions to proceed. The catalase enzyme (found in blood) lowers the. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it. Experiment Using Catalysts.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Experiment Using Catalysts The catalase enzyme (found in blood) lowers the. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. In this catalase and hydrogen peroxide experiment, we will. Experiment Using Catalysts.
From www.vernier.com
The of Hydrogen Peroxide > Experiment 12 from Advanced Chemistry with Vernier Experiment Using Catalysts In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. Catalysts provide an alternative route for reactions to proceed. A catalyst gets reactions started and makes them happen faster by. The catalase enzyme (found in blood) lowers the. A catalyst is a substance. Experiment Using Catalysts.
From www.youtube.com
Demonstration of a Simple Catalyst Experiment YouTube Experiment Using Catalysts Catalysts provide an alternative route for reactions to proceed. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. In this experiment,. Experiment Using Catalysts.
From apbiologyfinal-katiep.weebly.com
Liver Enzyme Lab Ap biology labs Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst gets reactions started and makes them happen faster by. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as. Experiment Using Catalysts.
From www.youtube.com
Potato Catalase Enzyme Experiment (UPDATE) YouTube Experiment Using Catalysts In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution. Experiment Using Catalysts.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing. Experiment Using Catalysts.
From www.mdpi.com
Catalysts Free FullText Dyesensitized Photocatalyst of Sepiolite for Organic Dye Degradation Experiment Using Catalysts Platinum metal catalysts can lower the activation energy to about 49 kj/mol. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Catalysts provide an alternative route for reactions to proceed. Use this demonstration to illustrate catalysis of the oxidation. Experiment Using Catalysts.
From www.youtube.com
Using Manganese Dioxide As a Catalyst to Hydrogen Peroxide into Water and Oxygen Gas Experiment Using Catalysts Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst gets reactions started and makes them happen faster by. In this experiment, you will extract. Experiment Using Catalysts.
From www.researchgate.net
(a) Schematics of Experimental Setup for ultrasound assisted synthesis... Download Scientific Experiment Using Catalysts Catalysts provide an alternative route for reactions to proceed. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Use. Experiment Using Catalysts.
From www.alamy.com
Full labelled diagram of the laboratory preparation of oxygen from Stock Vector Art Experiment Using Catalysts Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. Catalysts provide an alternative route for reactions to proceed. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. A catalyst is a substance that speeds up. Experiment Using Catalysts.
From scitechdaily.com
Science Made Simple What Are Catalysts? Experiment Using Catalysts The catalase enzyme (found in blood) lowers the. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. A catalyst gets reactions started and makes them happen faster by. A catalyst is a substance that speeds up a chemical reaction,. Experiment Using Catalysts.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Experiment Using Catalysts Catalysts provide an alternative route for reactions to proceed. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. A. Experiment Using Catalysts.
From www.researchgate.net
ROP monitoring experiments using zinc catalysts (color online). Download Scientific Diagram Experiment Using Catalysts A catalyst gets reactions started and makes them happen faster by. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. The catalase enzyme (found in blood) lowers the. Use this demonstration to illustrate catalysis of the oxidation of potassium. Experiment Using Catalysts.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction. Experiment Using Catalysts.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Chemistry Chemical Experiment Using Catalysts Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under. Experiment Using Catalysts.
From smartcatalyst.ir
Various Methods Of Preparing Iron Catalysts Experiment Using Catalysts In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. The catalase enzyme (found in blood) lowers the. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In this experiment, you will. Experiment Using Catalysts.
From www.mdpi.com
Catalysts Free FullText Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides Experiment Using Catalysts In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. A catalyst gets reactions started and makes them happen faster by. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and. Experiment Using Catalysts.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Experiment Using Catalysts Catalysts provide an alternative route for reactions to proceed. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the. Experiment Using Catalysts.
From www.mdpi.com
Catalysts Free FullText New Method of Determining Parameters for of Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning. Experiment Using Catalysts.
From www.youtube.com
Experiment Effect of Catalyst on Reaction Rate YouTube Experiment Using Catalysts Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. A catalyst gets reactions started and makes them happen. Experiment Using Catalysts.
From www.mdpi.com
Catalysts Free FullText Photocatalytic Hydrogen Production Role of Sacrificial Reagents on Experiment Using Catalysts Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. The catalase enzyme (found in blood) lowers the. A catalyst gets reactions started and makes them happen faster by. Catalysts provide an alternative route for reactions to proceed.. Experiment Using Catalysts.
From www.researchgate.net
6 Schematic diagram of hydrothermal method of synthesis. Download Scientific Diagram Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalase enzyme (found in blood) lowers the. Catalysts provide an alternative route for reactions to proceed.. Experiment Using Catalysts.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Organic Chemistry C6.2o Cracking Experiment Using Catalysts The catalase enzyme (found in blood) lowers the. Catalysts provide an alternative route for reactions to proceed. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. A catalyst is a substance that speeds up a chemical reaction, or lowers. Experiment Using Catalysts.
From borates.today
Catalytic Cracking Processes With Boron Catalyst Borates Today Experiment Using Catalysts In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. The catalase enzyme (found in blood) lowers the. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied. Experiment Using Catalysts.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 3.2.6 Practical Effect of Catalysts on Rate of Reaction翰林国际教育 Experiment Using Catalysts A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst gets reactions started and makes them happen faster by. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. Catalysts provide an alternative route for reactions to. Experiment Using Catalysts.
From www.tes.com
Catalysts lesson and experiment Teaching Resources Experiment Using Catalysts Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalysts provide an alternative route for reactions to proceed. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. The catalase enzyme (found in blood) lowers the. In this experiment, students compare the rate of reaction between iron (iii) nitrate. Experiment Using Catalysts.
From www.youtube.com
Fast Catalyst experiment beats student YouTube Experiment Using Catalysts Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. In this experiment, students compare the rate of reaction between iron (iii) nitrate solution and sodium thiosulfate solution when different transition metal ions are used as catalysts. Platinum metal catalysts can lower the activation energy to about 49 kj/mol. Catalysts. Experiment Using Catalysts.
From edu.rsc.org
Cracking hydrocarbons in liquid paraffin with a catalyst Experiment RSC Education Experiment Using Catalysts Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. In this catalase and hydrogen peroxide experiment, we will discover. Experiment Using Catalysts.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Experiment Using Catalysts In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by causing chemical. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts provide an alternative route for reactions to proceed. In this experiment, students compare the rate of reaction between. Experiment Using Catalysts.
From www.slideshare.net
1.4 rate of reaction(1.2d)...biology Experiment Using Catalysts Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In. Experiment Using Catalysts.
From www.researchgate.net
Control experiments. a Control experiment using Ni(II) catalyst. b... Download Scientific Diagram Experiment Using Catalysts A catalyst gets reactions started and makes them happen faster by. The catalase enzyme (found in blood) lowers the. In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Platinum metal catalysts can lower the activation energy to about 49. Experiment Using Catalysts.
From www.youtube.com
Demonstration of a Catalyst YouTube Experiment Using Catalysts In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Enzymes play a vital role in the chemical reactions that take place in our body, by functioning as a catalyst. In this experiment, students compare the rate of reaction between. Experiment Using Catalysts.
From www.researchgate.net
The leaching experiment for ZFOEG catalyst of benzylation reaction. Download Scientific Diagram Experiment Using Catalysts Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The catalase enzyme (found in blood) lowers the. Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide. In this catalase and hydrogen peroxide experiment, we will discover how enzymes act as catalysts by. Experiment Using Catalysts.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Experiment Using Catalysts In this experiment, you will extract the enzyme catalase from fresh (or frozen) liver, use it to break down hydrogen peroxide, and test the activity of catalase under different conditions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Use this demonstration to illustrate catalysis of the oxidation of. Experiment Using Catalysts.