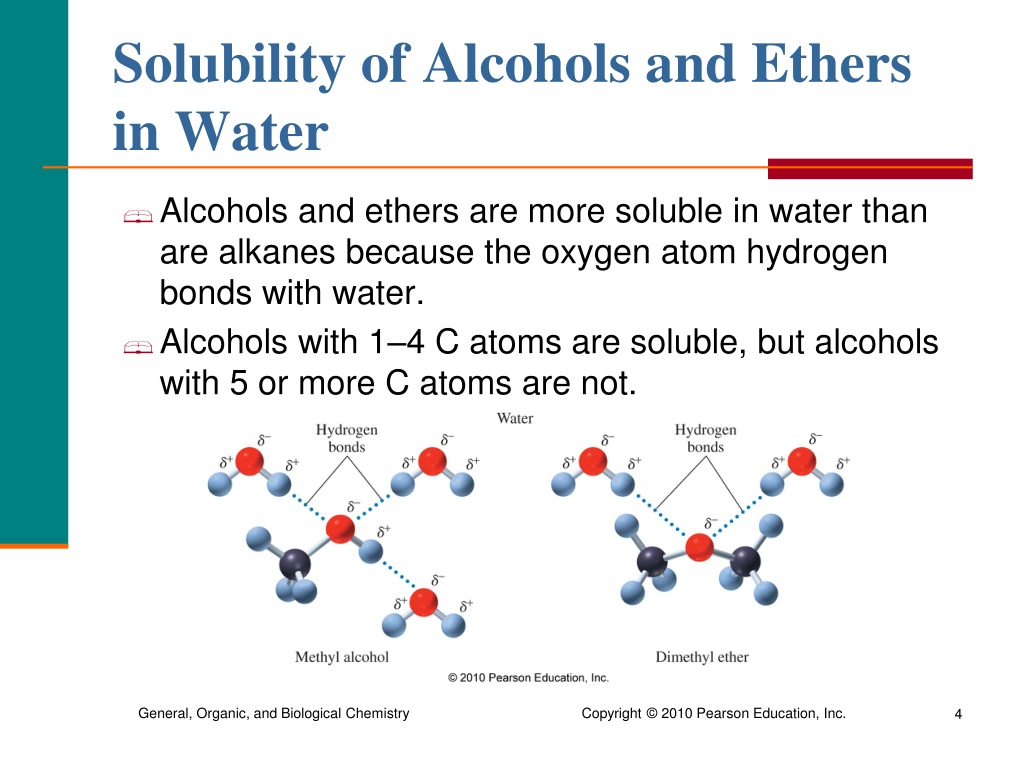
Is Phenol Soluble In Water . Mixing the two in any proportion generates a single solution. Phenol is able to interact with water. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Solubility of alcohols in water. Anything with a charged group (eg. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Small alcohols are completely soluble in water; Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring.
from www.slideserve.com
Solubility of alcohols in water. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Anything with a charged group (eg. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Phenol is able to interact with water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be.
PPT Chapter 13 Alcohols, Phenols, and Thiols PowerPoint Presentation ID9344627
Is Phenol Soluble In Water Anything with a charged group (eg. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; Phenol is able to interact with water. Anything with a charged group (eg. Solubility of alcohols in water. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be.
From physicalpharmacy2013.blogspot.com
PHYSICAL PHARMACY PRACTICAL 2 Determine the solubility curve for phenol and water Is Phenol Soluble In Water Anything with a charged group (eg. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. Solubility of alcohols in water. Learn about the solubility, boiling point,. Is Phenol Soluble In Water.
From physicalpharmacy1424.blogspot.com
Physical Pharmacy Practical Practical 2 Phase Diagram Mutual Solubility Curve for Phenol and Is Phenol Soluble In Water Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. Phenol is able to interact with water. Solubility of alcohols in water. B) phenol is more soluble. Is Phenol Soluble In Water.
From kadence-yersbloghamilton.blogspot.com
Is Phenol Soluble in Water Is Phenol Soluble In Water Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Mixing the two in any proportion generates a single solution. Phenol is able to interact with water. It is moderately. Is Phenol Soluble In Water.
From slideplayer.com
Phenols. ppt download Is Phenol Soluble In Water Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Phenol is able to interact with water. Solubility of alcohols in water. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. It is moderately soluble in water due to. Is Phenol Soluble In Water.
From www.scribd.com
Water Phenol PDF Solubility Phase (Matter) Is Phenol Soluble In Water Anything with a charged group (eg. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. B) phenol is more soluble in water than cyclohexanol because of. Is Phenol Soluble In Water.
From www.researchgate.net
Phase diagram of the phenol3water system under atmospheric pressure.... Download High Is Phenol Soluble In Water It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Phenol. Is Phenol Soluble In Water.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 Is Phenol Soluble In Water B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Anything with a charged group (eg. Mixing the two in any proportion generates a single solution. Ammonium, carboxylate, phosphate) is almost certainly. Is Phenol Soluble In Water.
From gbu-presnenskij.ru
Water Phenol PDF Solubility Phase (Matter), 58 OFF Is Phenol Soluble In Water Solubility of alcohols in water. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Small alcohols are completely soluble in water; Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless. Is Phenol Soluble In Water.
From www.researchgate.net
Solubility of phenol and its derivatives in water [28,29]. Download Scientific Diagram Is Phenol Soluble In Water Mixing the two in any proportion generates a single solution. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. B) phenol is more soluble in water than cyclohexanol because of the more. Is Phenol Soluble In Water.
From www.slideserve.com
PPT Phenol PowerPoint Presentation, free download ID3560996 Is Phenol Soluble In Water Phenol is able to interact with water. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Small alcohols are completely soluble in water; B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Anything with a charged group (eg. Mixing the. Is Phenol Soluble In Water.
From netsolwater.com
Why is it necessary to remove phenols from wastewater Is Phenol Soluble In Water B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Phenol dissolves to give. Is Phenol Soluble In Water.
From slideplayer.com
13.1 Alcohols, Phenols, Thiols, and Ethers ppt download Is Phenol Soluble In Water Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Anything with a charged group (eg. Mixing the two in any proportion generates a single solution. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Learn about the solubility, boiling point,. Is Phenol Soluble In Water.
From www.bartleby.com
Answered 4.) Phenol Why slightly soluble in… bartleby Is Phenol Soluble In Water It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Mixing the two in any proportion generates a single solution. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Anything with a charged group (eg. Phenol is able to interact with. Is Phenol Soluble In Water.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 Is Phenol Soluble In Water B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Phenol is able to interact with water. Mixing the two in any proportion generates a single solution. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Learn about the solubility, boiling point, acidity and. Is Phenol Soluble In Water.
From mutualsolubilitycurve20163b.blogspot.com
Lab Report for Experiment 3b MUTUAL SOLUBILITY CURVE FOR PHENOL AND WATER Is Phenol Soluble In Water B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Small. Is Phenol Soluble In Water.
From www.numerade.com
SOLVED Question 29 According to the following mutual solubility curve, the critical solution Is Phenol Soluble In Water Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will. Is Phenol Soluble In Water.
From www.numerade.com
Both phenol and cyclohexanol are only slightly soluble in water. Account for the fact that Is Phenol Soluble In Water Mixing the two in any proportion generates a single solution. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Small alcohols are completely soluble in water; Phenol is able to interact with water. Solubility of alcohols in water. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility. Is Phenol Soluble In Water.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross out what does not apply Is Phenol Soluble In Water It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Phenol is able to interact with water. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent. Is Phenol Soluble In Water.
From peterkruwwatson.blogspot.com
Is Phenol Soluble in Water PeterkruwWatson Is Phenol Soluble In Water Small alcohols are completely soluble in water; Solubility of alcohols in water. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Phenol is able to interact with water. Anything with a charged group (eg. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case. Is Phenol Soluble In Water.
From www.semanticscholar.org
Solubility of Phenolic Compounds in Pure Water and Alcohols with FTIR and DFT Study Semantic Is Phenol Soluble In Water Mixing the two in any proportion generates a single solution. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Solubility of alcohols in water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. It is moderately soluble. Is Phenol Soluble In Water.
From slideplayer.com
Chemistry Department, College of Science, King Saud University ppt download Is Phenol Soluble In Water Small alcohols are completely soluble in water; Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Mixing the two in any proportion generates a single solution. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Phenol is able to. Is Phenol Soluble In Water.
From www.numerade.com
SOLVED Classification Physical Properties of Alcohols and Phenols Unknown Alcohol pKa Soluble Is Phenol Soluble In Water Solubility of alcohols in water. Small alcohols are completely soluble in water; Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. Phenol is able to interact with water. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. B). Is Phenol Soluble In Water.
From www.fishersci.com
Phenol Red, Water Soluble, ACS, Spectrum Chemical Fisher Scientific Is Phenol Soluble In Water Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Anything with. Is Phenol Soluble In Water.
From www.numerade.com
SOLVED Text Physical Properties of Alcohols and Phenols Alcohol pH Soluble in Water? Ethanol 7 Is Phenol Soluble In Water Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Small alcohols are completely soluble in water; Phenol dissolves to give a 9.3 percent solution in water, compared with. Is Phenol Soluble In Water.
From chemistrypubs.com
Phenol hybridization and its solubility Chemistrupubs Is Phenol Soluble In Water Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Solubility. Is Phenol Soluble In Water.
From farmasireport.blogspot.com
Physicochemical Properties of Drugs Mutual Solubility Curve for Phenol and Water Is Phenol Soluble In Water B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Anything with a charged. Is Phenol Soluble In Water.
From www.youtube.com
PhenolWater System, Chemistry Lecture Sabaq.pk YouTube Is Phenol Soluble In Water Solubility of alcohols in water. Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. B) phenol is more soluble in water than cyclohexanol because of the. Is Phenol Soluble In Water.
From brainly.in
phenol water system with diagram Brainly.in Is Phenol Soluble In Water Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. Anything with a charged group (eg. It is moderately soluble in water due to its ability to. Is Phenol Soluble In Water.
From fyoqykvxc.blob.core.windows.net
Phenol Solubility In Water Temperature at David Hartzell blog Is Phenol Soluble In Water Phenol is soluble in water due to intermolecular hydrogen bonding, but its solubility decreases with the size of the aryl group. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most. Is Phenol Soluble In Water.
From www.researchgate.net
Watersoluble phenols (WSP) content in the soil at different... Download Scientific Diagram Is Phenol Soluble In Water Anything with a charged group (eg. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. Phenol dissolves to give a 9.3 percent solution in water, compared with a. Is Phenol Soluble In Water.
From www.slideserve.com
PPT Chapter 13 Alcohols, Phenols, and Thiols PowerPoint Presentation ID9344627 Is Phenol Soluble In Water Learn about the solubility, boiling point, acidity and chirality of phenols, the organic compounds containing benzene ring bonded to a hydroxyl group. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Anything with a charged group (eg. Small alcohols are completely soluble in water; Mixing the two in any proportion generates a. Is Phenol Soluble In Water.
From www.numerade.com
I need a conclusion of properties of alcohols and phenols lab experimental results using Is Phenol Soluble In Water Solubility of alcohols in water. Small alcohols are completely soluble in water; Anything with a charged group (eg. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Phenol is. Is Phenol Soluble In Water.
From fyoqykvxc.blob.core.windows.net
Phenol Solubility In Water Temperature at David Hartzell blog Is Phenol Soluble In Water It is moderately soluble in water due to its ability to form hydrogen bonds with the water. B) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Solubility of alcohols in water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will. Is Phenol Soluble In Water.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 Is Phenol Soluble In Water Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Ammonium, carboxylate, phosphate) is almost certainly water soluble, unless has a vary large nonpolar group, in which case it will most likely be. It is moderately soluble in water due to its ability to form hydrogen bonds with the water.. Is Phenol Soluble In Water.
From slideplayer.com
Fundamentals of Organic Chemistry ppt download Is Phenol Soluble In Water Small alcohols are completely soluble in water; Anything with a charged group (eg. It is moderately soluble in water due to its ability to form hydrogen bonds with the water. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Learn about the solubility, boiling point, acidity and chirality of. Is Phenol Soluble In Water.