Copper Ii Nitrate Sodium Hydroxide Balanced Equation . Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! See examples of copper, sodium,. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter an equation of an ionic chemical equation and press the balance button. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. The balanced equation will be calculated along with the. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enhanced with ai, our expert help has broken.
from www.youtube.com
Enhanced with ai, our expert help has broken. See examples of copper, sodium,. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Enter an equation of an ionic chemical equation and press the balance button. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients.
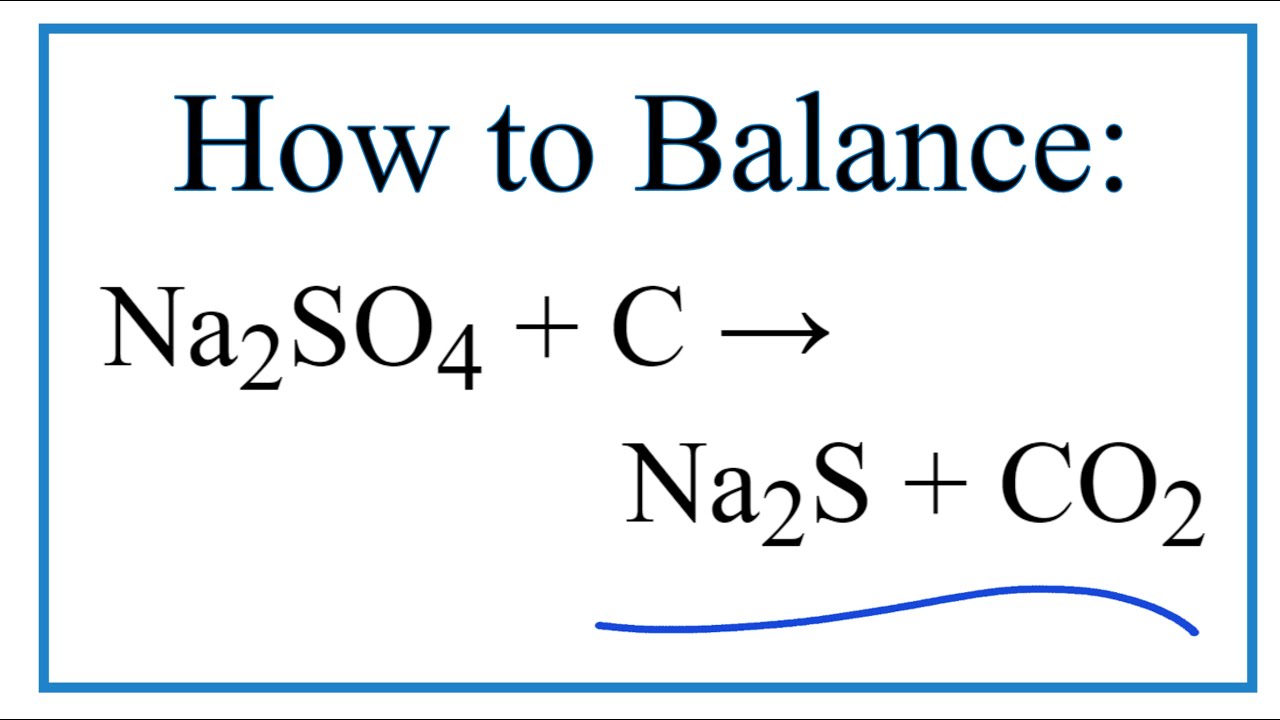
How to Balance Na2SO4 + C = Na2S + CO2 YouTube
Copper Ii Nitrate Sodium Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. See examples of copper, sodium,. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Enhanced with ai, our expert help has broken. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. See examples of copper, sodium,. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 1. Write balanced chemical equations for each Copper Ii Nitrate Sodium Hydroxide Balanced Equation See examples of copper, sodium,. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Balanced equation for. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Nitrate Sodium Hydroxide Balanced Equation In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. See examples of copper, sodium,. Enhanced with ai, our expert help has broken. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Learn how to balance chemical equations. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enhanced with ai, our expert help has broken. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter an equation of an ionic chemical equation and press the balance button. See examples of copper, sodium,. Balanced equation for copper (ii) nitrate and sodium hydroxide your. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved onsənd The product of the following word equation Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. See examples of copper, sodium,. Enhanced with ai, our expert help has broken. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. The balanced equation will. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED REACTION5COPPER SULFATE(CuSO,)AND SODIUM HYDROXIDE(NaOH) (Write balanced equation and Copper Ii Nitrate Sodium Hydroxide Balanced Equation In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.slideserve.com
PPT REACTION PREDICTION PreAP CHEMISTRY PowerPoint Presentation, free download ID3025342 Copper Ii Nitrate Sodium Hydroxide Balanced Equation In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter an equation of an ionic chemical equation and press the balance button. Balanced equation for copper. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of copper(II) nitrate with Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. See examples of copper, sodium,. Enter a chemical equation and press the balance button. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
2. Copper Nitrate to Copper Hydroxide (Cu Again Lab) YouTube Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Enter a chemical equation and press the balance button to get the balanced equation and the type. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED DUENL2QE Write " the complete, balanced equation for each of the following reactions (3 Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. See examples of copper, sodium,. Enhanced with ai, our expert help has broken. Enter an equation of. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for K2S + Cu(NO3)2 = KNO3 + CuS YouTube Copper Ii Nitrate Sodium Hydroxide Balanced Equation The balanced equation will be calculated along with the. Enhanced with ai, our expert help has broken. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of.. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Balance Na2SO4 + C = Na2S + CO2 YouTube Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enhanced with ai, our expert help has broken. Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter a chemical equation and press the balance button to get the balanced equation and the type. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDWrite complete, balanced equations for each of the following reactions. (a) Sodium metal Copper Ii Nitrate Sodium Hydroxide Balanced Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. See examples of copper, sodium,. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! Enhanced. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED 1. When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. In the second reaction. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.slideshare.net
Chemical equations Copper Ii Nitrate Sodium Hydroxide Balanced Equation Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. When carbon dioxide is. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
06 Copper nitrate and Sodium hydroxide YouTube Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. See examples of copper, sodium,. The balanced equation will be calculated along with the. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Learn how to. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. See examples of copper, sodium,. Enhanced with ai, our expert help has broken. In the second reaction the sodium hydroxide reacted with the. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Observations... Course Hero Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. The balanced equation will be calculated along with the. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Enter an equation of an ionic chemical equation and. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Observations... Course Hero Copper Ii Nitrate Sodium Hydroxide Balanced Equation The balanced equation will be calculated along with the. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Enhanced with ai, our expert help has broken. Enter an equation of an ionic chemical equation and press the balance button. Learn how to balance chemical equations using the law of conservation. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.bartleby.com
Answered copper(II) nitrate + sodium hydroxide>… bartleby Copper Ii Nitrate Sodium Hydroxide Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 2. Copper (II) Hydroxide From Copper (II) Nitrate Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Enter an equation of an ionic chemical equation. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper nitrate+Nitrogen dioxide Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button.. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Copper (II) nitrate and sodium hydrogen carbonate react according to the following Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the reaction of copper metal with Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Balanced. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper Ii Nitrate Sodium Hydroxide Balanced Equation In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Balanced. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Observations... Course Hero Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The equation above indicates that one. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium hydroxide (NaOH)? Cu(NO3)2 Copper Ii Nitrate Sodium Hydroxide Balanced Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. See examples of copper, sodium,. Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
Copper (II) nitrate Sodium phosphate Molecular equation Complete ionic equation Net ionic Copper Ii Nitrate Sodium Hydroxide Balanced Equation See examples of copper, sodium,. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Water And Sodium Hydroxide Tessshebaylo Copper Ii Nitrate Sodium Hydroxide Balanced Equation In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Enter an equation of an ionic chemical equation and press the balance button. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. When carbon dioxide is. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Aqueous solutions of copper (II) nitrate and sodium Copper Ii Nitrate Sodium Hydroxide Balanced Equation The balanced equation will be calculated along with the. See examples of copper, sodium,. Enter a chemical equation and press the balance button to get the balanced equation and the type of reaction. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Learn how to. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Word Equation Balanced Chemical Equation When solid copper reacts with aqueous silver Copper Ii Nitrate Sodium Hydroxide Balanced Equation The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! Enter an equation. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved VI. Write formula equations and balance with Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enhanced with ai, our expert help has broken. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. Enter a chemical equation and press the balance button to get the balanced equation and. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Formula for Copper (II) nitrate YouTube Copper Ii Nitrate Sodium Hydroxide Balanced Equation Enhanced with ai, our expert help has broken. See examples of copper, sodium,. Learn how to balance chemical equations using the law of conservation of mass and the method of coefficients. The balanced equation will be calculated along with the. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED a. What is the net ionic equation for silver nitrate + calcium carbonate? b. What is Copper Ii Nitrate Sodium Hydroxide Balanced Equation The balanced equation will be calculated along with the. The equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole of. Enter an equation of an ionic chemical equation and press the balance button. Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go!. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Writing chemical equations copper(II) nitrate + ammonium carbonate → copper(II Copper Ii Nitrate Sodium Hydroxide Balanced Equation Balanced equation for copper (ii) nitrate and sodium hydroxide your solution’s ready to go! The balanced equation will be calculated along with the. In the second reaction the sodium hydroxide reacted with the copper(ii)nitrate and produced sodium nitrate and copper(ii) hydroxide, the. See examples of copper, sodium,. The equation above indicates that one mole of solid copper is reacting with. Copper Ii Nitrate Sodium Hydroxide Balanced Equation.