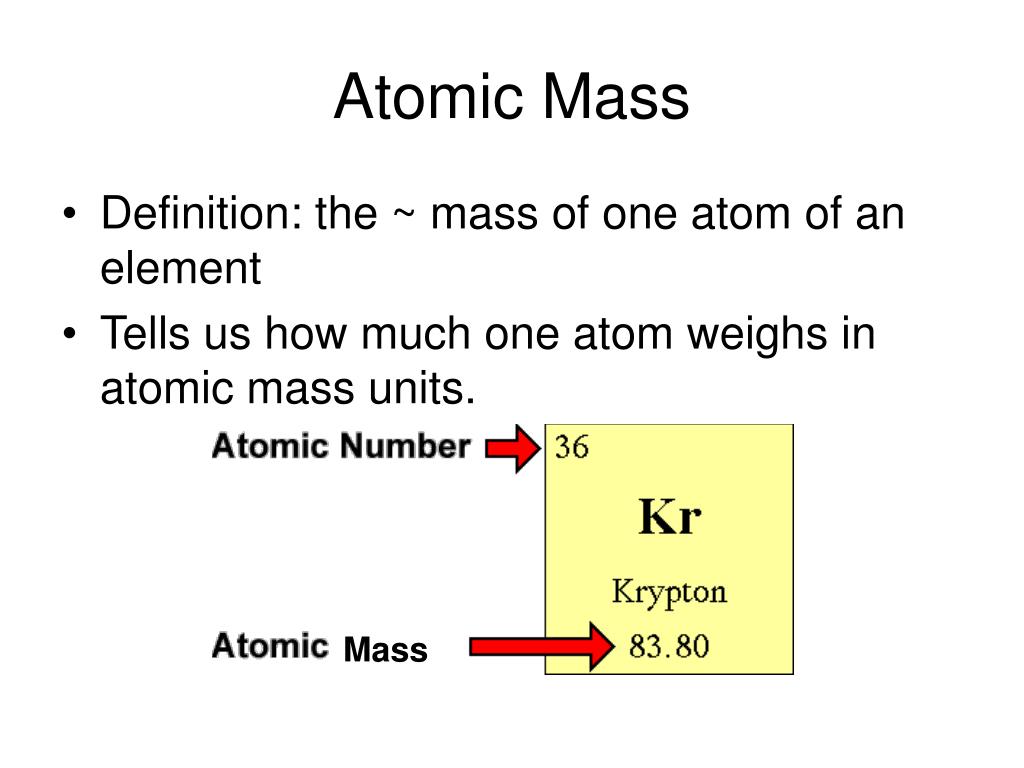
Define Atomic Mass Time . Atomic mass, the quantity of matter contained in an atom of an element. What do we mean by a. It is a weighted average because different isotopes have different masses. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. An atomic mass unit is 1/12th of the mass of a 12 c atom. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. Atomic mass indicates the size of an atom.
from www.slideserve.com
It is a weighted average because different isotopes have different masses. What do we mean by a. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass indicates the size of an atom. An atomic mass unit is 1/12th of the mass of a 12 c atom. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element.
PPT Organization of The Periodic Table PowerPoint Presentation, free
Define Atomic Mass Time What do we mean by a. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. An atomic mass unit is 1/12th of the mass of a 12 c atom. What do we mean by a. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. It is a weighted average because different isotopes have different masses. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. Atomic mass indicates the size of an atom. Atomic mass, the quantity of matter contained in an atom of an element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes.
From www.tes.com
Relative Atomic Mass OCR A Level Teaching Resources Define Atomic Mass Time Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. It is a weighted average because different isotopes have different masses. To. Define Atomic Mass Time.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID5318691 Define Atomic Mass Time An atomic mass unit is 1/12th of the mass of a 12 c atom. It is a weighted average because different isotopes have different masses. What do we mean by a. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass, which is also known as. Define Atomic Mass Time.
From www.politicalfunda.com
Atomic mass Atomic mass definition, Units, & Facts Define Atomic Mass Time An atomic mass unit is 1/12th of the mass of a 12 c atom. What do we mean by a. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. It is a weighted average because different isotopes have different masses. Atomic mass, which is also known as. Define Atomic Mass Time.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Define Atomic Mass Time Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass indicates the size of an atom. What do we mean by a. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance. Define Atomic Mass Time.
From www.slideserve.com
PPT Relative atomic mass A r PowerPoint Presentation, free download Define Atomic Mass Time Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. What do we mean by a. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. An atomic. Define Atomic Mass Time.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Define Atomic Mass Time The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. Atomic mass indicates the size of an atom. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass,. Define Atomic Mass Time.
From www.youtube.com
Relative atomic mass YouTube Define Atomic Mass Time An atomic mass unit is 1/12th of the mass of a 12 c atom. It is a weighted average because different isotopes have different masses. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. To solve this dilemma, we. Define Atomic Mass Time.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID Define Atomic Mass Time It is a weighted average because different isotopes have different masses. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass indicates the size of an atom. Atomic mass, the quantity of matter contained in an atom of an element. To solve this dilemma, we define. Define Atomic Mass Time.
From www.slideserve.com
PPT Atomic Mass and Intro to the Mole PowerPoint Presentation, free Define Atomic Mass Time The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes.. Define Atomic Mass Time.
From www.wikihow.com
How to Find Average Atomic Mass 8 Steps (with Pictures) wikiHow Define Atomic Mass Time Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass indicates the size of an atom. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally. Define Atomic Mass Time.
From www.studypool.com
SOLUTION Chemistry define atomic mass and atomic number classxi 2 Define Atomic Mass Time To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. It is a weighted average because different isotopes have different masses. What do we mean by a. Atomic mass indicates the size of an atom. An atomic mass unit is 1/12th of the mass of a 12 c. Define Atomic Mass Time.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Define Atomic Mass Time Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass indicates the size of an atom. An atomic mass unit is 1/12th of the mass of a 12 c atom. The atomic mass is an average of an. Define Atomic Mass Time.
From www.youtube.com
Relative Atomic Mass and Atomic Mass Unit (amu) Fundamental of Define Atomic Mass Time Atomic mass indicates the size of an atom. Atomic mass, the quantity of matter contained in an atom of an element. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes. Define Atomic Mass Time.
From www.slideserve.com
PPT Chapter 7 Chemical Formula Relationships PowerPoint Presentation Define Atomic Mass Time The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. Atomic mass indicates the size of an atom. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. An atomic mass unit is 1/12th of the mass of a 12 c atom.. Define Atomic Mass Time.
From www.politicalfunda.com
Atomic mass Atomic mass definition, Units, & Facts Define Atomic Mass Time An atomic mass unit is 1/12th of the mass of a 12 c atom. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. Atomic mass, which is. Define Atomic Mass Time.
From byjus.com
Atomic Mass And Molecular Mass Definition Difference Mass spectrometry Define Atomic Mass Time To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. It is a weighted average because different isotopes have different masses. Atomic mass indicates the size of an atom. An atomic mass unit is 1/12th of the mass of a 12 c atom. The atomic mass of an. Define Atomic Mass Time.
From www.tutoroot.com
Indepth Guide for Atomic Mass and Atomic Number Tutoroot Define Atomic Mass Time To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. An atomic mass unit is 1/12th of the mass of a 12 c atom. It is a weighted average because. Define Atomic Mass Time.
From www.teachoo.com
Definition, How to caluclate Atomic mass Teachoo Science Define Atomic Mass Time The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass indicates the size of an atom. The atomic mass of an element is a weighted average of the. Define Atomic Mass Time.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Define Atomic Mass Time The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. It is a weighted average because different isotopes have different masses. What do we mean by a. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the. Define Atomic Mass Time.
From en.wikipedia.org
Atomic mass Wikipedia Define Atomic Mass Time What do we mean by a. An atomic mass unit is 1/12th of the mass of a 12 c atom. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. To solve this dilemma, we define the atomic mass as. Define Atomic Mass Time.
From studylib.net
relative atomic mass Define Atomic Mass Time An atomic mass unit is 1/12th of the mass of a 12 c atom. Atomic mass indicates the size of an atom. Atomic mass, the quantity of matter contained in an atom of an element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. It is a weighted average because different. Define Atomic Mass Time.
From www.slideshare.net
What is Atomic Mass? Define Atomic Mass Time The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using. Define Atomic Mass Time.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow Define Atomic Mass Time Atomic mass indicates the size of an atom. An atomic mass unit is 1/12th of the mass of a 12 c atom. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic mass of an element is a. Define Atomic Mass Time.
From www.creative-chemistry.org.uk
Relative atomic mass Creative Chemistry Define Atomic Mass Time The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. What do we mean by a. Atomic mass, the quantity of matter contained in an atom of an element. An atomic mass unit is 1/12th of the mass of a 12 c atom. The atomic mass of an element is. Define Atomic Mass Time.
From www.youtube.com
Relative Atomic Mass How to Find Relative Atomic Mass of an Element Define Atomic Mass Time Atomic mass indicates the size of an atom. Atomic mass, the quantity of matter contained in an atom of an element. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. The atomic mass of an element is a weighted average of the masses of the isotopes that. Define Atomic Mass Time.
From utedzz.blogspot.com
Labeled Periodic Table Mass Numbers Periodic Table Timeline Define Atomic Mass Time It is a weighted average because different isotopes have different masses. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. What do we mean by. Define Atomic Mass Time.
From www.wikihow.com
3 Ways to Calculate Atomic Mass wikiHow Define Atomic Mass Time The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. It is a weighted average because different isotopes have different masses. An atomic mass unit is 1/12th of the mass of a 12 c atom. Atomic mass indicates the size of an atom. What do we mean by a. Atomic. Define Atomic Mass Time.
From www.youtube.com
Atomic Weight and Average Atomic Mass Chemistry Tutorial YouTube Define Atomic Mass Time The atomic mass of an element is a weighted average of the masses of the isotopes that compose an element. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass indicates the size of an atom. What do we mean by a. The atomic mass of. Define Atomic Mass Time.
From www.slideserve.com
PPT Atomic Mass and Intro to the Mole PowerPoint Presentation, free Define Atomic Mass Time It is a weighted average because different isotopes have different masses. An atomic mass unit is 1/12th of the mass of a 12 c atom. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. The atomic mass of an element is the weighted average of the masses. Define Atomic Mass Time.
From www.thoughtco.com
Difference Between Atomic Mass and Mass Number Define Atomic Mass Time Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. It is a weighted average because different isotopes have different masses. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. What. Define Atomic Mass Time.
From slideplayer.com
Chapter 2 Atoms and Molecules ppt download Define Atomic Mass Time The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass indicates the size of an atom. It is a weighted average because different isotopes have different masses. The atomic mass of an element is a weighted average of the masses of the isotopes that compose an. Define Atomic Mass Time.
From www.geeksforgeeks.org
Atomic Mass Table of First 30 Elements Define Atomic Mass Time Atomic mass indicates the size of an atom. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes. The atomic mass of an element is a weighted average of the. Define Atomic Mass Time.
From www.slideserve.com
PPT Organization of The Periodic Table PowerPoint Presentation, free Define Atomic Mass Time The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. Atomic mass, the quantity of matter contained in an atom of an element. An atomic mass unit is 1/12th of the mass of a 12 c atom. What do we mean by a. Atomic mass indicates the size. Define Atomic Mass Time.
From www.biologyonline.com
Unified atomic mass unit Definition and Examples Biology Online Define Atomic Mass Time Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass indicates the size of an atom. The atomic mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope of that element. An atomic mass unit is 1/12th of the mass of a 12 c atom. Atomic mass, which. Define Atomic Mass Time.
From www.slideserve.com
PPT ATOMS & THE PERIODIC TABLE PowerPoint Presentation, free download Define Atomic Mass Time Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. It is a weighted average because different isotopes have different masses. What do we mean by a. Atomic mass indicates the size of an atom. An atomic mass unit is. Define Atomic Mass Time.