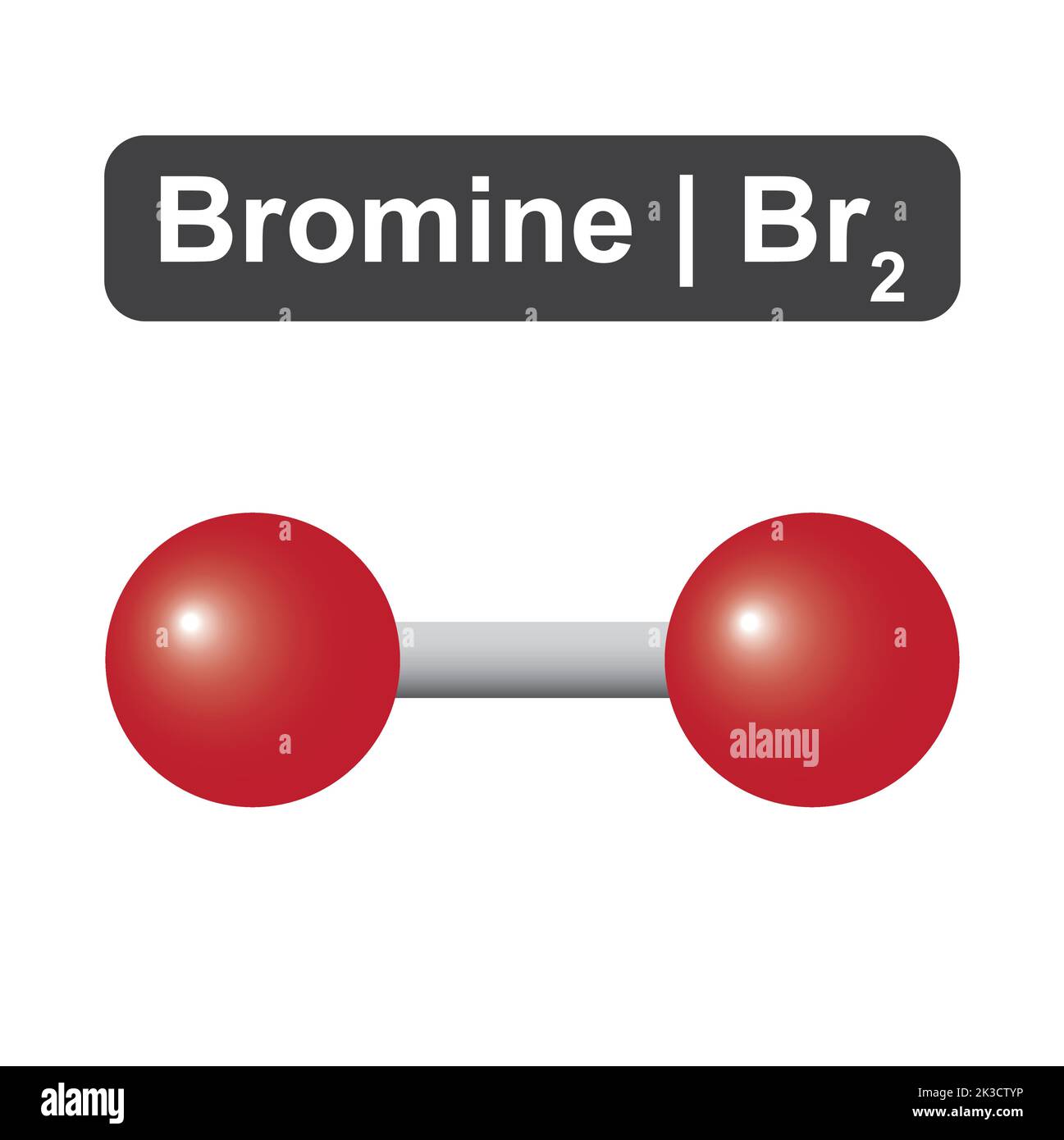
Is Bromine Diatomic . Bromine is a liquid at standard conditions and has the. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Pure bromine is diatomic, br 2. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn about its chemical and physical properties, reactions,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. It forms diatomic molecules with. Bromine is a reddish brown liquid and a member of the halogen elements.
from www.alamy.com
It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Pure bromine is diatomic, br 2. Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is a liquid at standard conditions and has the. Learn about its chemical and physical properties, reactions,. Bromine is a reddish brown liquid and a member of the halogen elements. It forms diatomic molecules with. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures.
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock
Is Bromine Diatomic Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Pure bromine is diatomic, br 2. Learn about its chemical and physical properties, reactions,. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is a liquid at standard conditions and has the. Learn about the diatomic elements, such as bromine, and how to write their formulas. It forms diatomic molecules with. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is a reddish brown liquid and a member of the halogen elements.
From favpng.com
Halogen Bromine Diatomic Molecule Liquid, PNG, 1100x790px, Halogen Is Bromine Diatomic Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. It forms diatomic molecules with. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn about its chemical and physical. Is Bromine Diatomic.
From www.dreamstime.com
Periodic Table Element Bromine Rendered Metal on Black on Black Stock Is Bromine Diatomic Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. It exists as diatomic molecules. Is Bromine Diatomic.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn about its chemical and physical properties, reactions,. It forms diatomic molecules with. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Bromine is one of the seven. Is Bromine Diatomic.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Is Bromine Diatomic It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Pure bromine is diatomic, br 2. Bromine is a reddish brown liquid and a member of the halogen elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a diatomic molecule because it is composed of. Is Bromine Diatomic.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy Is Bromine Diatomic It forms diatomic molecules with. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine. Is Bromine Diatomic.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Is Bromine Diatomic Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is a reddish brown liquid and a member of the halogen elements. Pure bromine is diatomic, br 2. It forms diatomic molecules with. Bromine is one of the seven. Is Bromine Diatomic.
From www.dreamstime.com
Diatomic Molecules Elements Diagram Colors Stock Vector Illustration Is Bromine Diatomic Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is a reddish brown liquid and a member of the halogen elements. It forms diatomic molecules with. Bromine is a liquid at standard conditions and has the. Pure bromine is diatomic, br 2. Bromine is a liquid at room temperature and. Is Bromine Diatomic.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Is Bromine Diatomic Pure bromine is diatomic, br 2. Learn about its chemical and physical properties, reactions,. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is a reddish brown liquid and a member of the halogen elements. It exists as diatomic molecules in all aggregation states and has a high electron affinity and. Is Bromine Diatomic.
From www.shutterstock.com
Diatomic Bromine Properties Chemical Compound Structure Stock Vector Is Bromine Diatomic Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Pure bromine. Is Bromine Diatomic.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Is Bromine Diatomic Learn about its chemical and physical properties, reactions,. Bromine is a reddish brown liquid and a member of the halogen elements. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Pure bromine is diatomic, br 2. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is the only. Is Bromine Diatomic.
From diagramlibraryclopped.z19.web.core.windows.net
Br2 Shape Of Molecule Is Bromine Diatomic Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn what diatomic elements are and which ones exist at. Is Bromine Diatomic.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is a liquid at standard conditions and has the. Learn about its. Is Bromine Diatomic.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Is Bromine Diatomic Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Learn about the diatomic elements, such as bromine, and how to write their formulas. It forms diatomic molecules with. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is a diatomic molecule because it is composed. Is Bromine Diatomic.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Is Bromine Diatomic It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Pure bromine is diatomic, br 2. Bromine is a diatomic molecule because it. Is Bromine Diatomic.
From www.shutterstock.com
Diatomic Bromine Properties Chemical Compound Structure Stock Vector Is Bromine Diatomic Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Learn about the diatomic elements, such as bromine, and how to write their formulas. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen,. Is Bromine Diatomic.
From techschematic.com
Understanding the Electron Dot Diagram for Bromine A Visual Is Bromine Diatomic Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is a reddish brown liquid and a member of the halogen elements. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is a liquid at standard conditions and has the. Bromine is a diatomic. Is Bromine Diatomic.
From www.alamy.com
Bromine chemical element, Sign with atomic number and atomic weight Is Bromine Diatomic It forms diatomic molecules with. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Pure bromine is diatomic, br 2. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Learn. Is Bromine Diatomic.
From pngtree.com
Atomic Properties Of Bromine Symbol Atomic Number And Atomic Mass Is Bromine Diatomic Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is the only nonmetallic element that is. Is Bromine Diatomic.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Is Bromine Diatomic Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Learn about its chemical and physical properties, reactions,. Bromine is a reddish brown liquid and a member of the halogen elements. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is one of the seven diatomic elements, along with. Is Bromine Diatomic.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Is Bromine Diatomic Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is a reddish brown liquid and a member of the halogen elements. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. It forms diatomic molecules with. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Learn about. Is Bromine Diatomic.
From www.youtube.com
Intermolecular Forces for Br2 (Diatomic Bromine) YouTube Is Bromine Diatomic Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn about its chemical and physical properties, reactions,. Pure bromine is diatomic, br 2. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is a diatomic molecule. Is Bromine Diatomic.
From www.numerade.com
Bromine is a halogen; one of the elements in the same column of the Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically. Is Bromine Diatomic.
From www.vecteezy.com
Bromine symbol. Chemical element of the periodic table. Vector Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. It forms diatomic molecules with. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Pure bromine is diatomic, br 2. Bromine is a diatomic molecule. Is Bromine Diatomic.
From www.dreamstime.com
Bromine Chemical 35 Element of Periodic Table. Molecule and Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is one of the seven diatomic elements, along with. Is Bromine Diatomic.
From www.alamy.com
Bromine molecular model Stock Vector Images Alamy Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. Pure bromine is diatomic, br 2. Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a liquid at standard conditions and has the. Learn what diatomic elements are. Is Bromine Diatomic.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Is Bromine Diatomic Bromine is a liquid at standard conditions and has the. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Pure bromine is diatomic, br 2. It exists as diatomic molecules in all aggregation states and has a. Is Bromine Diatomic.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy Is Bromine Diatomic It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is the only nonmetallic element that is liquid. Is Bromine Diatomic.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Is Bromine Diatomic Bromine is a liquid at standard conditions and has the. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. It forms diatomic molecules with. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Learn about the diatomic elements, such as bromine, and. Is Bromine Diatomic.
From www.dreamstime.com
Bromine Chemical Element Symbol on Light Background Stock Illustration Is Bromine Diatomic Bromine is the only nonmetallic element that is liquid at ordinary temperatures. It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Bromine is a reddish brown liquid and a member of the halogen elements. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Bromine is. Is Bromine Diatomic.
From www.dreamstime.com
Bromine, Br, Periodic Table Element Stock Illustration Illustration Is Bromine Diatomic It exists as diatomic molecules in all aggregation states and has a high electron affinity and a pungent odor. Learn about the diatomic elements, such as bromine, and how to write their formulas. Learn about its chemical and physical properties, reactions,. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. Bromine is one of the. Is Bromine Diatomic.
From www.shutterstock.com
Diatomic Bromine Molecular Structure Formula Periodic Stock Vector Is Bromine Diatomic Bromine is a reddish brown liquid and a member of the halogen elements. Bromine is a liquid at standard conditions and has the. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is a liquid at room temperature. Is Bromine Diatomic.
From www.anyrgb.com
Pblock, homonuclear Molecule, bromine Dioxide, iodine127, diatomic Is Bromine Diatomic Learn about its chemical and physical properties, reactions,. Bromine is a reddish brown liquid and a member of the halogen elements. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Learn what diatomic elements are and which ones exist. Is Bromine Diatomic.
From mungfali.com
Aufbau Diagram For Bromine Is Bromine Diatomic Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Bromine is a liquid at room temperature and belongs to the halogen group of nonmetals. Bromine is a reddish brown liquid and a member of. Is Bromine Diatomic.
From imgbin.com
Halogen Bromine Diatomic Molecule Liquid PNG, Clipart, Bromine, Bromine Is Bromine Diatomic Learn about its chemical and physical properties, reactions,. Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is a diatomic molecule because it is composed of two bromine atoms that are chemically bonded together. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. It forms diatomic molecules with. Pure bromine is. Is Bromine Diatomic.
From mungfali.com
Aufbau Diagram For Bromine Is Bromine Diatomic Bromine is one of the seven diatomic elements, along with hydrogen, nitrogen, oxygen, fluorine, chlorine, and iodine. Learn what diatomic elements are and which ones exist at ordinary temperatures and pressures. It forms diatomic molecules with. Learn about its chemical and physical properties, reactions,. Learn about the diatomic elements, such as bromine, and how to write their formulas. Bromine is. Is Bromine Diatomic.