Standard Enthalpy Of Formation Mgcl2 Aq . Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+.
from www.numerade.com
A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+.
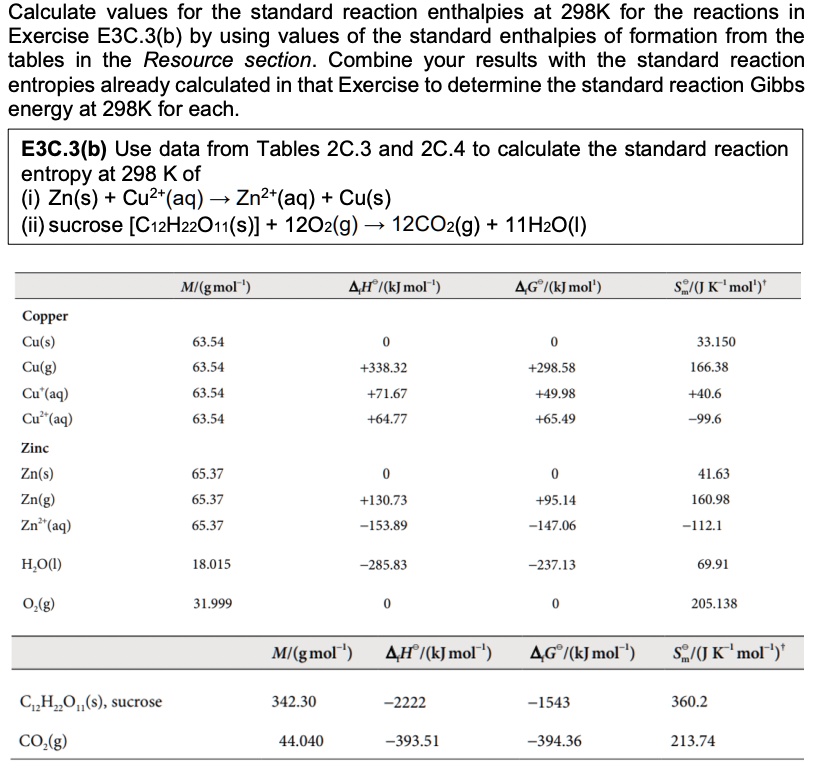
SOLVED Calculate values for the standard reaction enthalpies at 298k
Standard Enthalpy Of Formation Mgcl2 Aq Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.coursehero.com
[Solved] The standard enthalpy of formation of MgCl2(s) is AfH = 641. Standard Enthalpy Of Formation Mgcl2 Aq Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Mgcl2 Aq Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation Mgcl2 Aq.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g). Standard Enthalpy Of Formation Mgcl2 Aq.
From www.toppr.com
The absolute enthalpy of neutralisation of the reaction will be MgO(s Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g). Standard Enthalpy Of Formation Mgcl2 Aq.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Mgcl2 Aq Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Using Hess's Law and the values for Standard Enthalpies of Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Calculate values for the standard reaction enthalpies at 298k Standard Enthalpy Of Formation Mgcl2 Aq Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Mgcl2 Aq Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from. Standard Enthalpy Of Formation Mgcl2 Aq.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Mgcl2 Aq Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.slideserve.com
PPT Lattice enthalpy PowerPoint Presentation ID6559902 Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Mgcl2 Aq A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.chegg.com
Reaction A) Mg(s) + 2HCl (aq) > H2(g) + MgCl2 (a... Standard Enthalpy Of Formation Mgcl2 Aq Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. Standard Enthalpy Of Formation Mgcl2 Aq.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Mgcl2 Aq Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. One of the most useful kinds of heats of reaction to measure and tabulate is the standard. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Text Magnesium oxide reacts with hydrochloric acid and Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
VIDEO solution Calculate the value of AH for the reaction VOu Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Calculate the enthalpy change per mole of magnesium and Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a. Standard Enthalpy Of Formation Mgcl2 Aq.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Mgcl2 Aq Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq) given Standard Enthalpy Of Formation Mgcl2 Aq Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.researchgate.net
MgCl2 properties. Reaction enthalpy data from [98]. Investigated Standard Enthalpy Of Formation Mgcl2 Aq Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Standand enthalpies of formation & standard entropies of common compounds substance state. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Using the table of standard entropies and enthalpies of Standard Enthalpy Of Formation Mgcl2 Aq One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh. Standard Enthalpy Of Formation Mgcl2 Aq.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Mgcl2 Aq Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g). Standard Enthalpy Of Formation Mgcl2 Aq.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Mgcl2 Aq A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.researchgate.net
Enthalpies of MgCl2 and ZnCl2 with temperature in PIM. Download Standard Enthalpy Of Formation Mgcl2 Aq Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of a substance, δho f, the enthalpy. Definition and explanation of the terms standard state and standard enthalpy. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.numerade.com
SOLVED Using Hess's Law and the values for Standard Enthalpies of Standard Enthalpy Of Formation Mgcl2 Aq Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.chegg.com
Part B. Enthalpy for Mg(s)+2HCl(aq)→MgCl2(aq)+H2( g) Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Mgcl2 Aq Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25 degrees celsius and one atom from elements in their stable form. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.youtube.com
The absolute enthalpy of neutralisation of the reactionMgO(s)+2HCl(aq Standard Enthalpy Of Formation Mgcl2 Aq Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values. Standard Enthalpy Of Formation Mgcl2 Aq.
From www.transtutors.com
(Solved) Hess's Law Mg(S) + 2HCl(Aq) — + MgCl2(Aq) + H20, — H2(G Standard Enthalpy Of Formation Mgcl2 Aq Mg (s) + 2 hcl (aq) → mgcl2 (aq) + h2 (g) heat of formation (δhform) values for: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. One of the most useful kinds of heats of reaction to measure and tabulate is the standard enthalpy of formation of. Standard Enthalpy Of Formation Mgcl2 Aq.