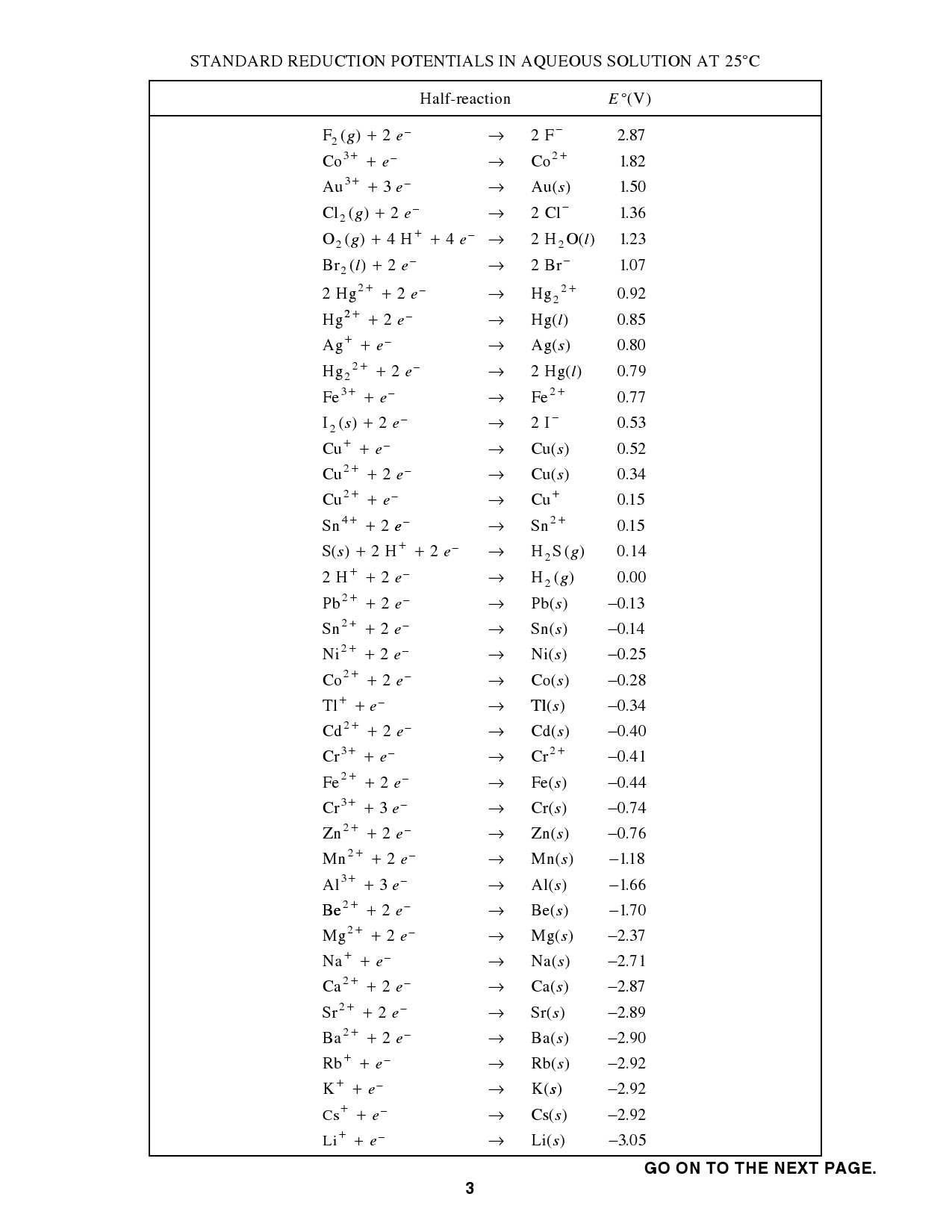
Standard Reduction Potential Element . 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Element, reaction equation and standard potential. Reduction reactions in acidic solution are written using h + in place of. Solids, gases, and liquids are identified; All other species are aqueous. The standard reduction potential is measured under standard conditions: 47 rows the table of standard redox potentials for total and inorganic chemistry contains: T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire.
from www.albertgural.com
The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Reduction reactions in acidic solution are written using h + in place of. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Solids, gases, and liquids are identified; The standard reduction potential is measured under standard conditions: Element, reaction equation and standard potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: All other species are aqueous.
Equations Albert Gural
Standard Reduction Potential Element The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Reduction reactions in acidic solution are written using h + in place of. Element, reaction equation and standard potential. All other species are aqueous. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential is measured under standard conditions: Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: Solids, gases, and liquids are identified;
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. The standard reduction potential is measured under standard conditions: 47 rows the table of standard redox potentials for total and inorganic chemistry contains: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode. Standard Reduction Potential Element.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID541675 Standard Reduction Potential Element Reduction reactions in acidic solution are written using h + in place of. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Use standard. Standard Reduction Potential Element.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Element Element, reaction equation and standard potential. All other species are aqueous. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h + in place of. 47 rows the table of standard redox. Standard Reduction Potential Element.
From www.slideserve.com
PPT Intersection 13 PowerPoint Presentation, free download ID567059 Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Element.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Element Element, reaction equation and standard potential. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. All other species are aqueous. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Reduction reactions in acidic solution are written using h. Standard Reduction Potential Element.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Element Use standard reduction potentials to determine the better oxidizing or. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Solids, gases, and liquids are identified; The standard reduction potential is measured under standard conditions: Reduction reactions in acidic solution are written using h + in place of. 372 rows the data. Standard Reduction Potential Element.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Solids, gases, and liquids are identified; Element, reaction equation and standard potential. All other species are aqueous. The standard reduction potential. Standard Reduction Potential Element.
From www.researchgate.net
1 Standard redox potentials involving lanthanide elements as dissolved Standard Reduction Potential Element Use standard reduction potentials to determine the better oxidizing or. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: Solids, gases, and liquids are identified; The standard reduction potential is measured under. Standard Reduction Potential Element.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Element The standard reduction potential is measured under standard conditions: Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. Reduction reactions in acidic solution are written using h + in place of. Element, reaction equation and standard potential.. Standard Reduction Potential Element.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Reduction Potential Element Reduction reactions in acidic solution are written using h + in place of. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: The standard reduction potential is measured under standard conditions: The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Solids,. Standard Reduction Potential Element.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID6102398 Standard Reduction Potential Element The standard reduction potential is measured under standard conditions: T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Element, reaction equation and standard potential. Use standard reduction potentials. Standard Reduction Potential Element.
From www.researchgate.net
Standard reduction potential (V vs Ag/AgCl) of different elements in Standard Reduction Potential Element All other species are aqueous. Element, reaction equation and standard potential. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. The standard reduction potential. Standard Reduction Potential Element.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Element Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h + in place of. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in. Standard Reduction Potential Element.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Element The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. All other species are aqueous. Element, reaction equation and standard potential. Reduction reactions in acidic. Standard Reduction Potential Element.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Element Reduction reactions in acidic solution are written using h + in place of. All other species are aqueous. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a. Standard Reduction Potential Element.
From www.slideserve.com
PPT 21 Electrochemistry PowerPoint Presentation, free download ID Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. The table is ordered such that the stronger (more reactive) reductants are at the top and. Standard Reduction Potential Element.
From www.numerade.com
SOLVED Table 2. Table of Selected Standard Reduction Potentials at 25Â Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Element, reaction equation and standard potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. All other species are aqueous. The standard reduction potential is the tendency for a chemical. Standard Reduction Potential Element.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential Element Use standard reduction potentials to determine the better oxidizing or. All other species are aqueous. Element, reaction equation and standard potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Reduction reactions in acidic solution are written using h + in place of. The standard reduction potential, also. Standard Reduction Potential Element.
From rayb78.github.io
Standard Reduction Potentials Chart Standard Reduction Potential Element Solids, gases, and liquids are identified; Use standard reduction potentials to determine the better oxidizing or. The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.. Standard Reduction Potential Element.
From labbyag.es
Reduction Potential Chart Labb by AG Standard Reduction Potential Element The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. 372. Standard Reduction Potential Element.
From www.slideserve.com
PPT Electrochemistry Part III Reduction Potentials PowerPoint Standard Reduction Potential Element 47 rows the table of standard redox potentials for total and inorganic chemistry contains: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Use standard reduction potentials to determine the better oxidizing or. Element, reaction equation and standard potential. The standard reduction potential is measured under standard conditions:. Standard Reduction Potential Element.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID5404690 Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Element, reaction equation and standard potential. 47 rows the table. Standard Reduction Potential Element.
From pveducation.org
Standard Potential PVEducation Standard Reduction Potential Element Solids, gases, and liquids are identified; The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Reduction reactions in acidic solution are written using. Standard Reduction Potential Element.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Element Solids, gases, and liquids are identified; All other species are aqueous. Reduction reactions in acidic solution are written using h + in place of. Element, reaction equation and standard potential. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Standard Reduction Potential Element.
From www.scribd.com
P1 Standard Reduction Potentials by Element PDF Manganese Chemistry Standard Reduction Potential Element 47 rows the table of standard redox potentials for total and inorganic chemistry contains: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential is measured under standard conditions: Use standard reduction potentials to determine the better oxidizing or. T = 298.15 k (25 °c,. Standard Reduction Potential Element.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. 47 rows the table of standard redox potentials for total and inorganic chemistry contains: The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. The standard reduction potential is. Standard Reduction Potential Element.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Element The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for. Standard Reduction Potential Element.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Element T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Solids, gases, and liquids are identified; Element, reaction equation and standard potential. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. All other species are aqueous. Use standard reduction. Standard Reduction Potential Element.
From www.slideserve.com
PPT ELECTROCHEMISTRY Chapter 21 PowerPoint Presentation, free Standard Reduction Potential Element 47 rows the table of standard redox potentials for total and inorganic chemistry contains: The standard reduction potential is measured under standard conditions: T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. All other species are aqueous. Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode. Standard Reduction Potential Element.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Reduction Potential Element Element, reaction equation and standard potential. Reduction reactions in acidic solution are written using h + in place of. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical species to acquire. Use standard reduction potentials to determine the better oxidizing or. The standard reduction potential is the tendency for. Standard Reduction Potential Element.
From ecampusontario.pressbooks.pub
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Element Element, reaction equation and standard potential. T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The table is ordered such that the stronger (more reactive) reductants are at the top. Standard Reduction Potential Element.
From www.albertgural.com
Equations Albert Gural Standard Reduction Potential Element The standard reduction potential is measured under standard conditions: T = 298.15 k (25 °c, or 77 °f), a unity activity (a = 1) for each ion. Reduction reactions in acidic solution are written using h + in place of. The standard reduction potential, also known as the standard electrode potential, is a measure of the tendency of a chemical. Standard Reduction Potential Element.
From vpia.org.vn
Standard Reduction Potentials made easy, reduction line Standard Reduction Potential Element 47 rows the table of standard redox potentials for total and inorganic chemistry contains: The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. Element, reaction equation and standard potential. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured. Standard Reduction Potential Element.
From www.tpsearchtool.com
Appendix 4 Standard Reduction Potentials First Year General Chemistry Standard Reduction Potential Element 47 rows the table of standard redox potentials for total and inorganic chemistry contains: All other species are aqueous. Element, reaction equation and standard potential. The standard reduction potential is measured under standard conditions: Solids, gases, and liquids are identified; The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at. Standard Reduction Potential Element.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Element 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential is measured under standard conditions: 47 rows the table of standard redox potentials. Standard Reduction Potential Element.