Bromine Electrons To Gain . Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms gain or lose electrons to form anions or cations, respectively. Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 16 gain two electrons and form ions with a 2− charge,. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms of group 17 gain one electron and form anions with a 1− charge;
from manuallistcantabank.z21.web.core.windows.net
However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms of group 17 gain one electron and form anions with a 1− charge; The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms of group 17 gain one electron and form anions with a 1− charge; The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms gain or lose electrons to form anions or cations, respectively. Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion.
Lewis Dot Diagram For Bromine
Bromine Electrons To Gain Atoms gain or lose electrons to form anions or cations, respectively. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 17 gain one electron and form anions with a 1− charge; Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms gain or lose electrons to form anions or cations, respectively.
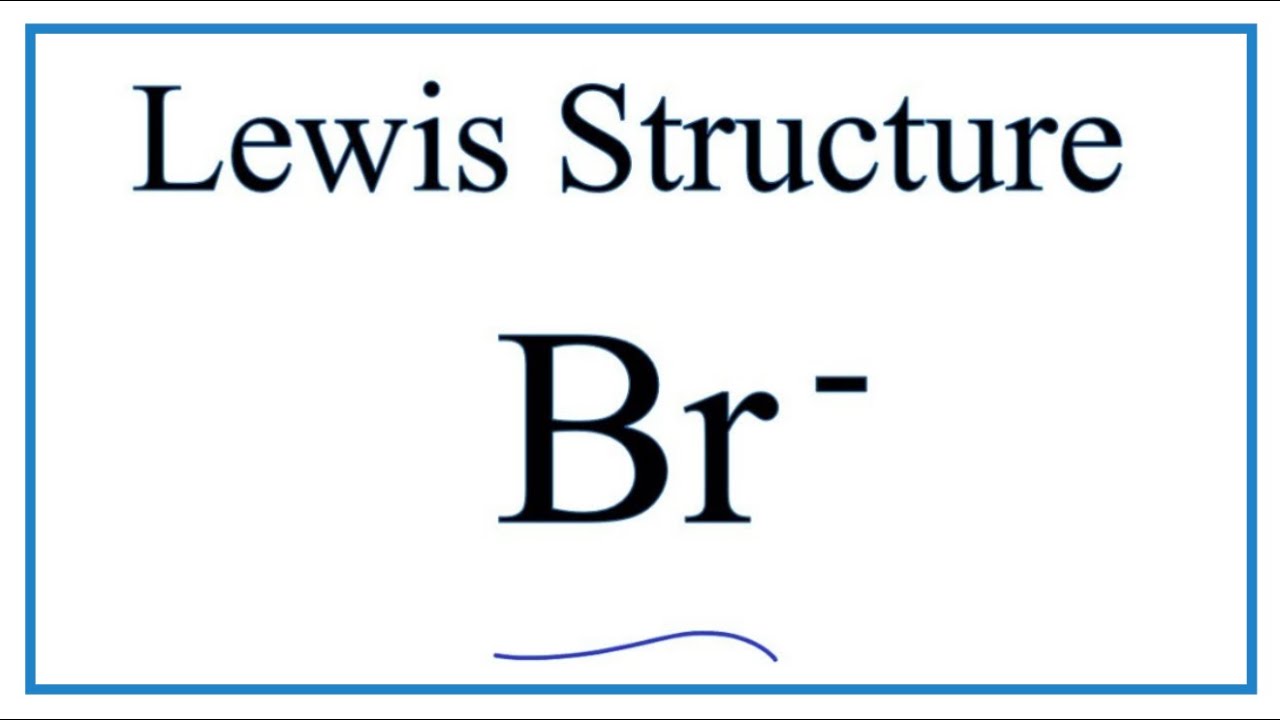
From www.numerade.com
SOLVEDWhy is bromine reduced in the following reaction (i.e., in what Bromine Electrons To Gain Atoms of group 16 gain two electrons and form ions with a 2− charge,. The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms of group 17 gain one electron and form anions with a 1− charge; Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p. Bromine Electrons To Gain.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Electrons To Gain Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Bromine has an electron configuration of 1s 2 2s. Bromine Electrons To Gain.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Electrons To Gain Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas,. Bromine Electrons To Gain.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Bromine Electrons To Gain The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms. Bromine Electrons To Gain.
From dokumen.tips
(PDF) WS, Ionic Bonding Edl Bonding WS Element Gain or Lose of Bromine Electrons To Gain However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms gain or lose electrons to form anions or cations, respectively. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 16. Bromine Electrons To Gain.
From www.numerade.com
SOLVED 0 / 1 pts Question 11 As per octet rule, bromine atom would Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of. Bromine Electrons To Gain.
From slideplayer.com
Review Review Cl1 Unstable Stable Stable ppt download Bromine Electrons To Gain Atoms of group 16 gain two electrons and form ions with a 2− charge,. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms gain or lose electrons to form anions or cations, respectively.. Bromine Electrons To Gain.
From www.youtube.com
The electron gain enthalpy (in \( \mathrm{kJ} / \mathrm{mol} \) ) of Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms of group 17 gain one electron. Bromine Electrons To Gain.
From slideplayer.com
Chemistry ppt download Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Bromine has an electron configuration. Bromine Electrons To Gain.
From www.numerade.com
SOLVED Select the number of electrons that each atom needs t0 gain Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. The primary decay mode of isotopes lighter than 79 br is electron capture. Bromine Electrons To Gain.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms of group 16 gain two. Bromine Electrons To Gain.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the. Bromine Electrons To Gain.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electrons To Gain Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms of group 17 gain one electron and form anions with a 1− charge; The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Bromine has 7 electrons in its outer shell and needs to. Bromine Electrons To Gain.
From lawsonjoyshebert.blogspot.com
Valence Electrons in Br LawsonjoysHebert Bromine Electrons To Gain Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 17 gain one electron and form anions with a 1−. Bromine Electrons To Gain.
From slideplayer.com
NonMetals. ppt download Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 17 gain one electron and form anions with a 1− charge; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms gain or lose electrons to form anions. Bromine Electrons To Gain.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; However, bromine is more likely to form anions because it gains one electron to complete. Bromine Electrons To Gain.
From einvoice.fpt.com.vn
Valence Electrons Definition, Role Examples Video Lesson, 51 OFF Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 16 gain two electrons and form ions with. Bromine Electrons To Gain.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Bromine Electrons To Gain However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms of group 17 gain one. Bromine Electrons To Gain.
From slideplayer.com
Unit 5 Ionic Bonding & Nomenclature ppt download Bromine Electrons To Gain Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms gain or lose electrons to form anions or cations, respectively. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of. Bromine Electrons To Gain.
From slideplayer.com
Review Review Cl1 Unstable Stable Stable ppt download Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 16 gain two electrons and form ions with. Bromine Electrons To Gain.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Electrons To Gain However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms gain or. Bromine Electrons To Gain.
From www.numerade.com
SOLVED Neutral bromine (Br2) can gain two electrons to the Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms of group 17 gain one electron and form anions with a 1− charge; The most stable bromine radioisotope. Bromine Electrons To Gain.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Electrons To Gain Atoms gain or lose electrons to form anions or cations, respectively. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2− charge,. Bromine has an electron configuration of 1s. Bromine Electrons To Gain.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms gain or lose electrons to form anions or cations, respectively. Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. The primary decay mode of isotopes lighter than 79 br is. Bromine Electrons To Gain.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Electrons To Gain Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms gain or lose electrons to form anions or cations, respectively.. Bromine Electrons To Gain.
From www.youtube.com
Explainhy the electron gain enthalpy of fluorine is less negative than Bromine Electrons To Gain However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2−. Bromine Electrons To Gain.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 17 gain. Bromine Electrons To Gain.
From studylib.net
By GAINING or LOSING electrons Bromine Electrons To Gain Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Atoms of. Bromine Electrons To Gain.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms of group 17 gain one electron and form anions with a 1− charge; Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. Atoms of group 16 gain. Bromine Electrons To Gain.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine Electrons To Gain Bromine has 7 electrons in its outer shell and needs to gain one electron to have a full outer shell and become an ion. However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s. Bromine Electrons To Gain.
From fyoekyjrv.blob.core.windows.net
Electron Configuration Of Bromine Long Form at Lowell Sadowski blog Bromine Electrons To Gain Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Bromine Electrons To Gain.
From www.chegg.com
Solved neutral bromine (Br2) can gain two electrons to Bromine Electrons To Gain The most stable bromine radioisotope is 77 br (t1/2 = 57.04 h). Atoms of group 17 gain one electron and form anions with a 1− charge; However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Atoms gain or lose electrons to form anions or cations, respectively. Atoms of group 17. Bromine Electrons To Gain.
From slideplayer.com
Unit 5 Ionic Bonding & Nomenclature ppt download Bromine Electrons To Gain Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge,. Atoms of group 16 gain two electrons and form ions with a 2− charge,. The primary decay mode. Bromine Electrons To Gain.
From slideplayer.com
Choice B Br2(l) Br2(g) 2 Br(g) 2 Br+(g) + 2e ppt download Bromine Electrons To Gain The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms of group 17 gain one electron and form anions with a 1− charge; However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. The most stable bromine radioisotope is 77 br (t1/2 =. Bromine Electrons To Gain.
From www.toppr.com
26. Use the following data to calculate Hº NaBr. A Hºfor sodium metal Bromine Electrons To Gain However, bromine is more likely to form anions because it gains one electron to complete its octet and attain. Atoms of group 16 gain two electrons and form ions with a 2− charge,. The primary decay mode of isotopes lighter than 79 br is electron capture to isotopes of selenium; Atoms gain or lose electrons to form anions or cations,. Bromine Electrons To Gain.