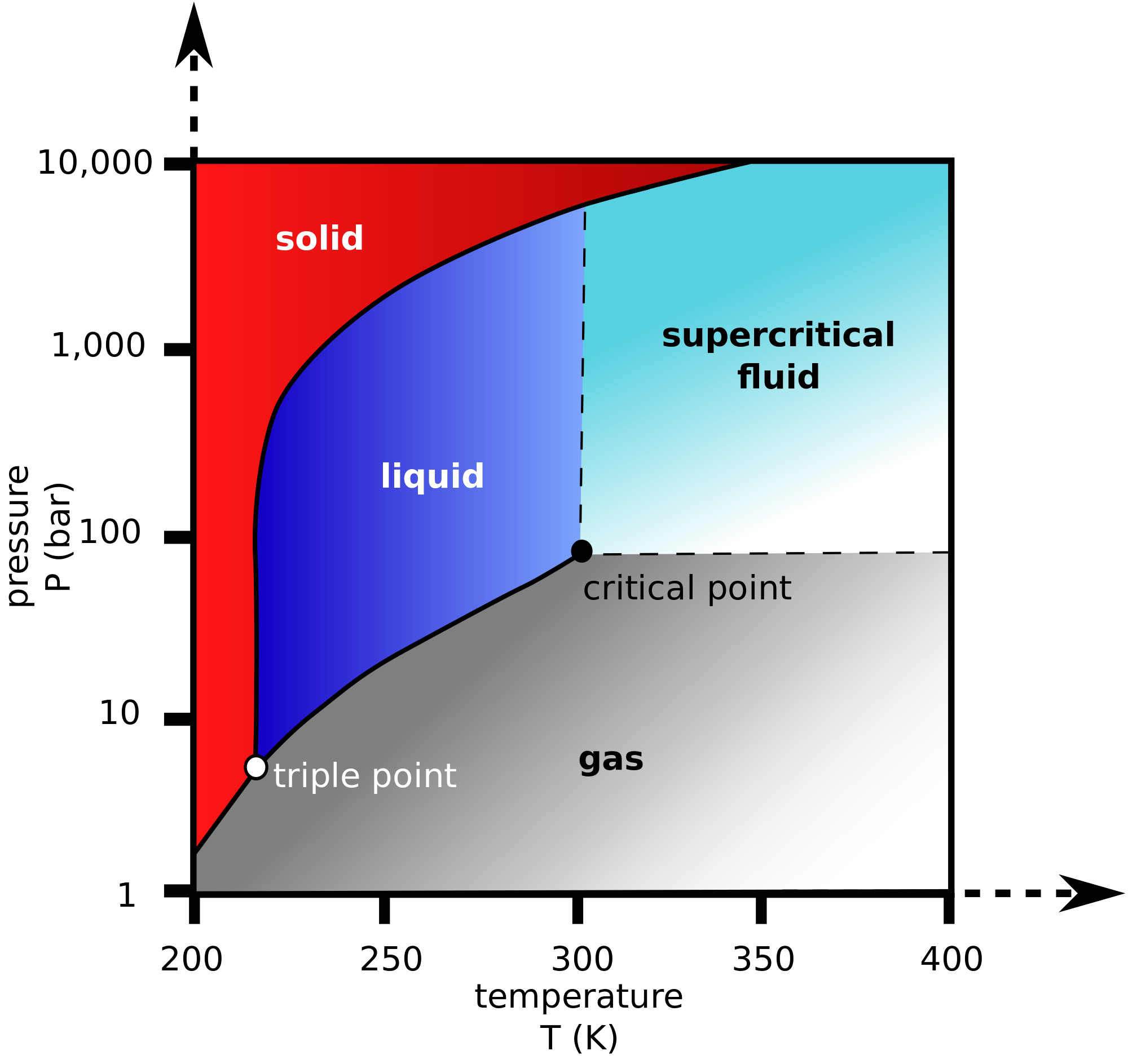
Does Pressure Affect Phase Change . Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. In this section, we will talk. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Find out the enthalpies of fusion, vaporization, and sublimation. Why does the pressure remain constant in the coexistence area (grey area)? Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid.
from socratic.org
Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Find out the enthalpies of fusion, vaporization, and sublimation. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Why does the pressure remain constant in the coexistence area (grey area)? In this section, we will talk. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c.
What is the relation between critical temperature and boiling point or
Does Pressure Affect Phase Change Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. In this section, we will talk. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Find out the enthalpies of fusion, vaporization, and sublimation. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Why does the pressure remain constant in the coexistence area (grey area)?
From philschatz.com
Phase Changes · Physics Does Pressure Affect Phase Change Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. In this section, we will talk. For instance, at atmospheric pressure, water melts at 0 °c and boils. Does Pressure Affect Phase Change.
From fyodaxjon.blob.core.windows.net
How Do Pressure Cookers Affect Phase Change In Water at Helen Schell blog Does Pressure Affect Phase Change In this section, we will talk. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. For instance, at atmospheric pressure, water melts at 0 °c. Does Pressure Affect Phase Change.
From www.freepik.com
Premium Vector Water States of matter Phase Change of State for Water Does Pressure Affect Phase Change Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Why does the pressure remain constant in the coexistence area (grey area)? Find out the enthalpies of fusion, vaporization, and sublimation. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due. Does Pressure Affect Phase Change.
From hxevlfnux.blob.core.windows.net
Why Does Pressure Affect Phase Change at Beth Evans blog Does Pressure Affect Phase Change Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such. Does Pressure Affect Phase Change.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Does Pressure Affect Phase Change Find out the enthalpies of fusion, vaporization, and sublimation. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Pressure affects phase transitions by altering the temperature. Does Pressure Affect Phase Change.
From www.facebook.com
The PMLP MEDL S1 KSA SemiFinals are here, and the competition is Does Pressure Affect Phase Change In this section, we will talk. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Learn about the six ways a substance can change between solid,. Does Pressure Affect Phase Change.
From ecgwaves.com
Ventricular PressureVolume Relationship Preload, Afterload, Stroke Does Pressure Affect Phase Change Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. In this section, we will talk. For instance, at atmospheric pressure, water melts at 0 °c and. Does Pressure Affect Phase Change.
From lavelle.chem.ucla.edu
melting CHEMISTRY COMMUNITY Does Pressure Affect Phase Change For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Learn about. Does Pressure Affect Phase Change.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Pressure Affect Phase Change Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due. Does Pressure Affect Phase Change.
From x-engineer.org
The states (phases) of matter (aggregation) Does Pressure Affect Phase Change Why does the pressure remain constant in the coexistence area (grey area)? Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Learn about the six ways a substance. Does Pressure Affect Phase Change.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Affect Phase Change Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Why does the pressure remain constant in the coexistence area (grey area)? Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Say we went from a. Does Pressure Affect Phase Change.
From socratic.org
What is the relation between critical temperature and boiling point or Does Pressure Affect Phase Change In this section, we will talk. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Find out the enthalpies of fusion, vaporization, and sublimation. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. For instance,. Does Pressure Affect Phase Change.
From socratic.org
Calculate the change in freezing point for ice if the pressure changes Does Pressure Affect Phase Change Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it. Does Pressure Affect Phase Change.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Affect Phase Change Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Why does the pressure remain constant in the coexistence area (grey area)? Learn how atmospheric pressure affects. Does Pressure Affect Phase Change.
From sciencenotes.org
States of Matter Does Pressure Affect Phase Change Why does the pressure remain constant in the coexistence area (grey area)? Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. For instance, at atmospheric pressure, water melts. Does Pressure Affect Phase Change.
From www.ck12.org
Phase Diagrams CK12 Foundation Does Pressure Affect Phase Change For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. In this section, we will talk. Find out the enthalpies of fusion, vaporization, and sublimation. Learn how atmospheric pressure affects the boiling point. Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 Does Pressure Affect Phase Change Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Find out the. Does Pressure Affect Phase Change.
From www.facebook.com
The PMLP MEDL S1 KSA SemiFinals are here, and the competition is Does Pressure Affect Phase Change For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Why does the pressure remain constant in the coexistence area (grey area)? Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Learn how energy changes when matter undergoes phase transitions, such as solid to. Does Pressure Affect Phase Change.
From hxeuornzy.blob.core.windows.net
How Does Pressure Affect Phase Change at Benjamin Goodwin blog Does Pressure Affect Phase Change Find out the enthalpies of fusion, vaporization, and sublimation. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Say we went from a gaseous state to. Does Pressure Affect Phase Change.
From www.researchgate.net
Photothermal effect, phase change, drugrelease behavior, and in vitro Does Pressure Affect Phase Change Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Find out the enthalpies of fusion, vaporization, and sublimation. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Say we went from a gaseous state to. Does Pressure Affect Phase Change.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Does Pressure Affect Phase Change In this section, we will talk. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another,. Does Pressure Affect Phase Change.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID6014910 Does Pressure Affect Phase Change Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and. Does Pressure Affect Phase Change.
From www.researchgate.net
Schematic illustration of the effect of similar intrathoracic pressures Does Pressure Affect Phase Change Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Find out the enthalpies of fusion, vaporization, and sublimation. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Why does the pressure remain constant in the coexistence area (grey area)? Learn about the. Does Pressure Affect Phase Change.
From www.unm.edu
BIOL 238 Class Notes Respiratory System Does Pressure Affect Phase Change In this section, we will talk. Find out the enthalpies of fusion, vaporization, and sublimation. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Why does. Does Pressure Affect Phase Change.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Does Pressure Affect Phase Change Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does not affect intermolecular forces. Why does the pressure remain constant in the coexistence area (grey area)? For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Find out the enthalpies of fusion, vaporization, and sublimation. Pressure affects phase. Does Pressure Affect Phase Change.
From chem.libretexts.org
13.4 Pressure and Temperature Effects on Solubility Chemistry LibreTexts Does Pressure Affect Phase Change Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Learn. Does Pressure Affect Phase Change.
From philschatz.com
The Process of Breathing · Anatomy and Physiology Does Pressure Affect Phase Change Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Learn how atmospheric pressure affects the boiling point and melting point of substances, and how it does. Does Pressure Affect Phase Change.
From www.thoughtco.com
List of Phase Changes Between States of Matter Does Pressure Affect Phase Change Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. In this section, we will talk. For instance, at atmospheric pressure, water melts at 0 °c and boils. Does Pressure Affect Phase Change.
From hxevlfnux.blob.core.windows.net
Why Does Pressure Affect Phase Change at Beth Evans blog Does Pressure Affect Phase Change Why does the pressure remain constant in the coexistence area (grey area)? Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. For instance, at atmospheric pressure, water melts at 0 °c and boils at 100 °c. Find out the enthalpies of fusion, vaporization, and sublimation. Say we. Does Pressure Affect Phase Change.
From www.logiota.com
Dalton's Law of Partial Pressure States of Matter Physical Does Pressure Affect Phase Change Learn how energy changes when matter undergoes phase transitions, such as solid to liquid, liquid to gas, and gas to solid. Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret. Does Pressure Affect Phase Change.
From hxeuornzy.blob.core.windows.net
How Does Pressure Affect Phase Change at Benjamin Goodwin blog Does Pressure Affect Phase Change Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature. Does Pressure Affect Phase Change.
From www.youtube.com
The Effect of Pressure Changes on Equilibrium YouTube Does Pressure Affect Phase Change Say we went from a gaseous state to a liquid state ($e\rightarrow a$), shouldn't the pressure increase due to the. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature. Does Pressure Affect Phase Change.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Does Pressure Affect Phase Change Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. In this section, we will talk. Learn how atmospheric pressure affects the boiling point and melting. Does Pressure Affect Phase Change.
From courses.lumenlearning.com
Phase Changes Physics Does Pressure Affect Phase Change In this section, we will talk. Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. Learn about the six ways a substance can change between solid, liquid, and gas phases, and how temperature and pressure affect phase. Find out the enthalpies of fusion, vaporization, and sublimation. Say. Does Pressure Affect Phase Change.
From circuitlistmisdoing99.z21.web.core.windows.net
Sound Frequency For High Blood Pressure Does Pressure Affect Phase Change Learn how a liquid can change to a gas at low temperatures or high pressures, and how to interpret a phase diagram. In this section, we will talk. Pressure affects phase transitions by altering the temperature at which substances change from one phase to another, such as from solid to. Why does the pressure remain constant in the coexistence area. Does Pressure Affect Phase Change.